Organosilicon compounds, production processes thereof, pressure-sensitive adhesive compositions containing the organosilicon compounds, self-adhesive polarizers and liquid crystal displays
a technology of organosilicon and organic compounds, applied in the field of organic compounds, can solve the problems of high potential risk of breaking the costly liquid cell, difficulty in increasing the size beyond 20 inches, and increasing the move toward larger panels, etc., to achieve excellent rework capability, excellent long-term durability, and excellent coordination property
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
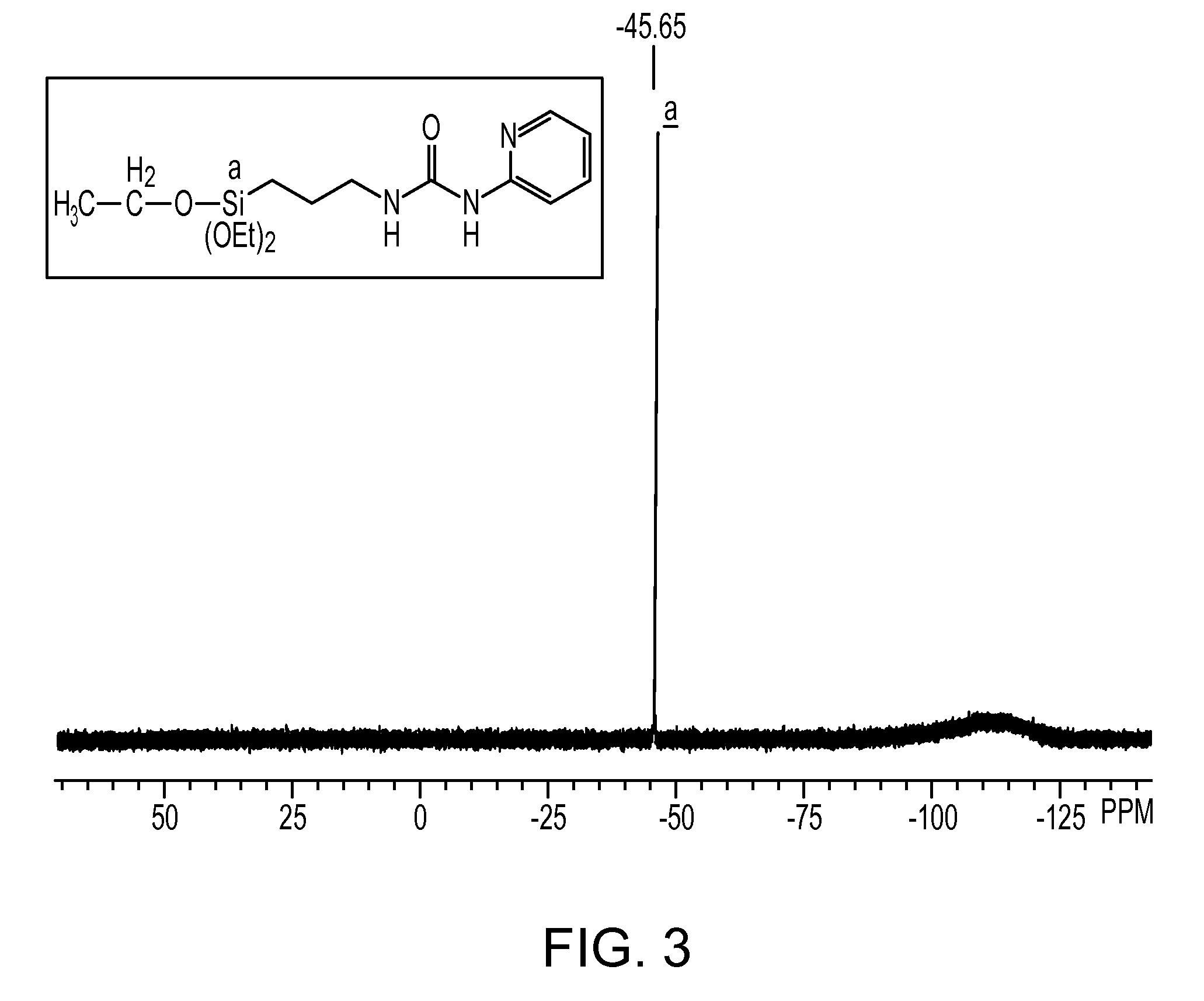
[0091]In a 1-L separable flask equipped with a stirrer, a reflux condenser, a dropping funnel and a thermometer, 31.4 g (0.33 mol) of 2-aminopyridine was placed, followed by charging of 150 g of tetrahydrofuran. The resultant mixture was stirred into a solution. Into the solution, 82.5 g (0.33 mol) of 3-isocyanatopropyltriethoxysilane was charged dropwise, and the thus-obtained mixture was stirred under heating at 70° C. for 4 hours. Subsequently, it is confirmed by IR measurement that an absorption peak attributable to the isocyanato group in the reactant 3-isocyanatopropyltriethoxy-silane had disappeared completely and instead, an absorption peak attributable to a urea bond had been formed, the reaction was determined to be completed. The solvent was then distilled off to obtain the reaction product, which was a pale yellow liquid and had a viscosity of 184 mm2 / s, a specific gravity of 1.094 and a refractive index of 1.4975. The reaction product was confirmed by GPC to be consiste...
synthesis example 2
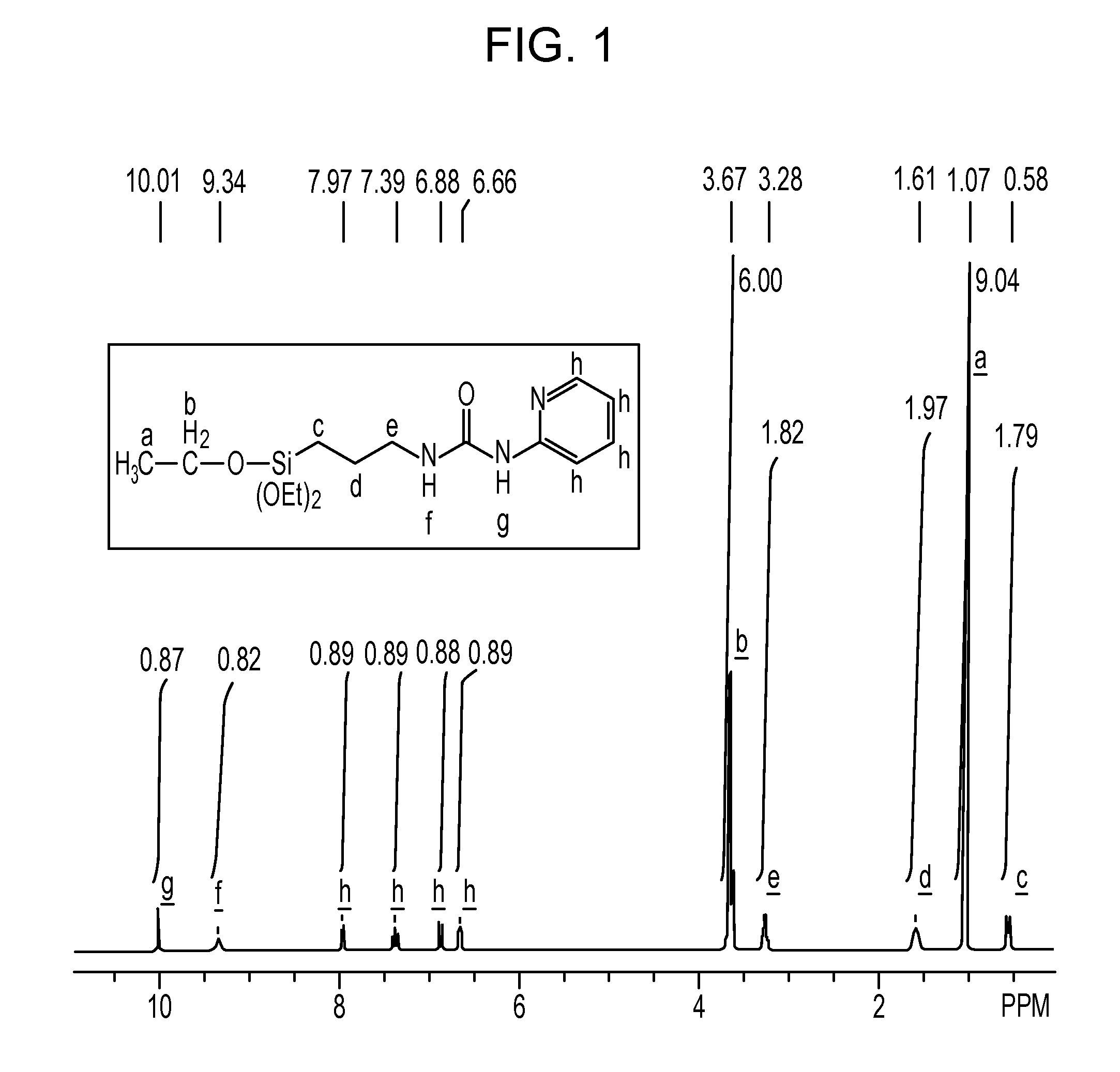
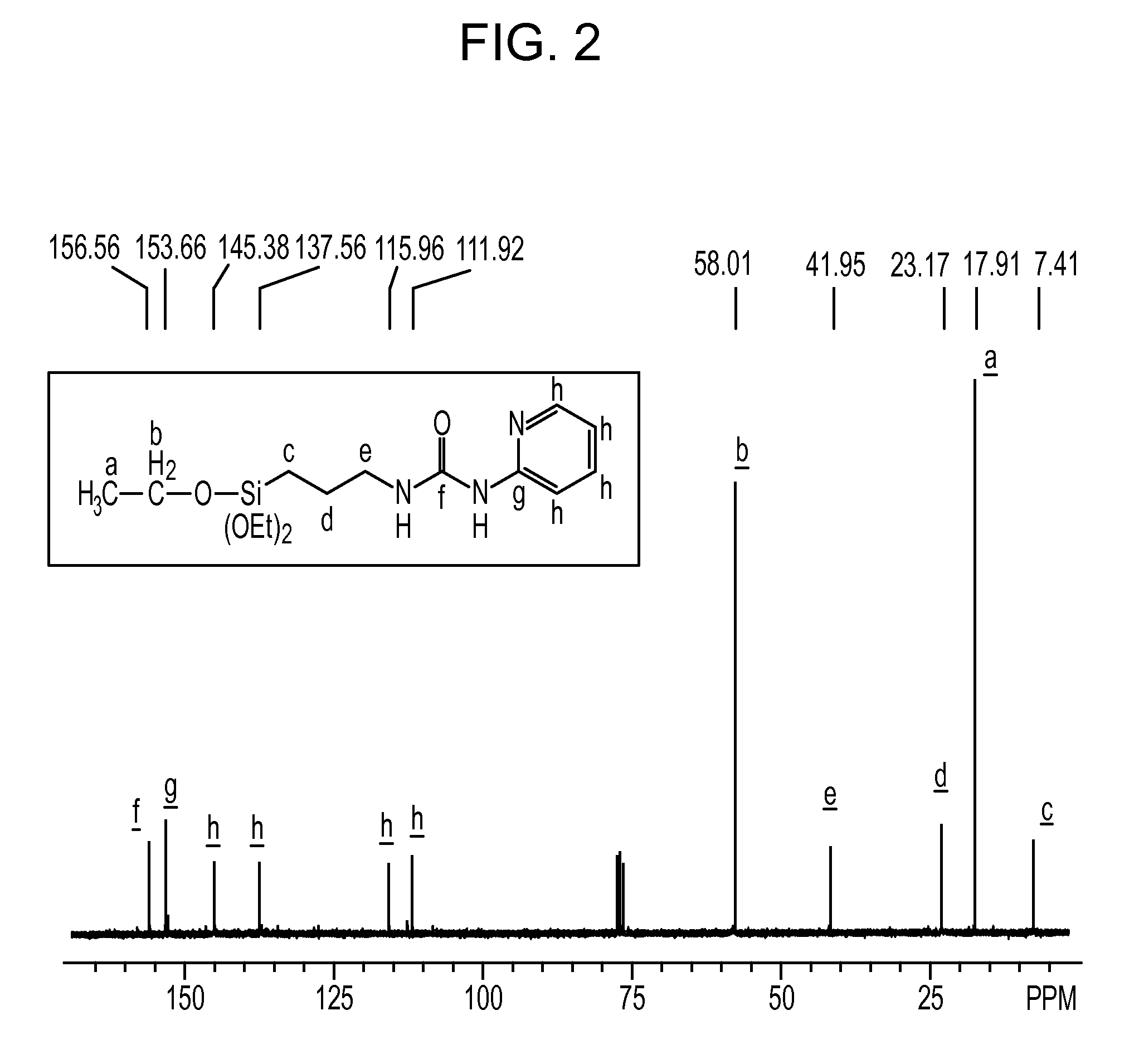
[0092]In a 1-L separable flask equipped with a stirrer, a reflux condenser, a dropping funnel and a thermometer, 31.7 g (0.33 mol) of 2-aminopyrimidine was placed, followed by charging of 150 g of tetrahydrofuran. The resultant mixture was stirred into a solution. Into the solution, 82.5 g (0.33 mol) of 3-isocyanatopropyltriethoxysilane was charged dropwise, and the thus-obtained mixture was stirred under heating at 70° C. for 4 hours. Subsequently, it is confirmed by IR measurement that an absorption peak attributable to the isocyanato group in the reactant 3-isocyanatopropyltriethoxy-silane had disappeared completely and instead, an absorption peak attributable to a urea bond had been formed, the reaction was determined to be completed. The solvent was then distilled off to obtain the reaction product, which was an orange solid. The reaction product was confirmed by NMR spectroscopy to have the below-described chemical structural formula (9). Its NMR spectral data are as follows:
[...
synthesis example 3
[0096]In a 1-L separable flask equipped with a stirrer, a reflux condenser, a dropping funnel and a thermometer, 89.4 g (0.75 mol) of 2-mercaptothiazoline and 1.3 g of dioctyltin oxide were placed, followed by charging of 300 g of ethyl acetate. The resultant mixture was stirred into a solution. Into the solution, 185.5 g (0.75 mol) of 3-isocyanatopropyl-triethoxysilane was charged dropwise, and the thus-obtained mixture was stirred under heating at 80° C. for 4 hours. Subsequently, it is confirmed by IR measurement that an absorption peak attributable to the isocyanato group in the reactant 3-isocyanatopropyltriethoxysilane had disappeared completely and instead, an absorption peak attributable to a thiourethane bond had been formed, the reaction was determined to be completed. The solvent was then distilled off to obtain the reaction product, which was a pale yellow liquid and had a viscosity of 38 mm2 / s, a specific gravity of 1.164 and a refractive index of 1.5218. The reaction p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| sizes | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com