Salts of physiologically active and psychoactive alkaloids and amines simultaneously exhibiting bioavailability and abuse resistance
a technology of physiologically active and psychoactive alkaloids and amines, which is applied in the field of pharmaceutically acceptable salts of alkaloids or amine containing compounds, can solve the problems of not getting the desired effect, increase the technical difficulty of isolating, inhibit and/or prevent the de-formulation of a drug product, and reduce the economic viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Phentermine Pamoate
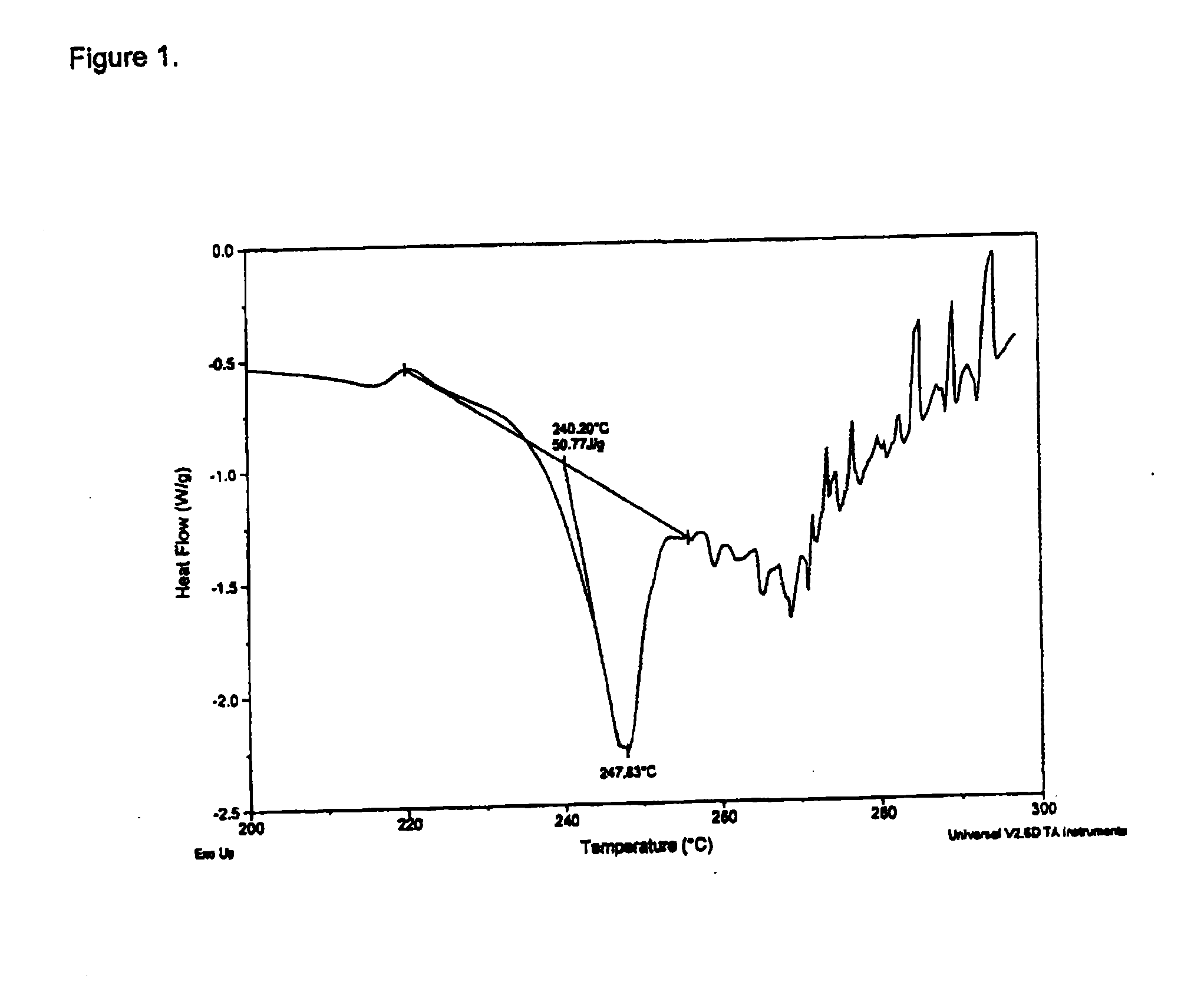
[0151]Phentermine HCl (37.3 g) was suspended in USP H2O (700.0 g) and stirred to achieve a solution (0.05 g / g) and a pH 5.3. The solution was transferred to a metered addition funnel. A solution of disodium pamoate (45.0 g) was prepared by dissolving in USP water (902.0 g) to give a clear solution at a pH 10.4. The solution was adjusted to about pH 9.4 with 0.2N HCl and clarified by solution filtration. At 20° C., the Phentermine HCl solution was added to the stirred disodium pamoate solution at a controlled rate over about 2.5 hours and the addition funnel rinsed with USP H2O (20.0 g) into the reaction mixture. The mixture was stirred for 1 h then warmed to 70° C. where more precipitation was observed. The mixture was heated from about 70° C. to 95° C. over about 1 h then cooled. Solids were collected by filtration and washed with USP H2O (3×200 g) and dried on a vacuum Büchner for about 3 h. The solids collected (83.5 g) were transferred to a dryi...
example 2
Preparation of Ephedrine Pamoate
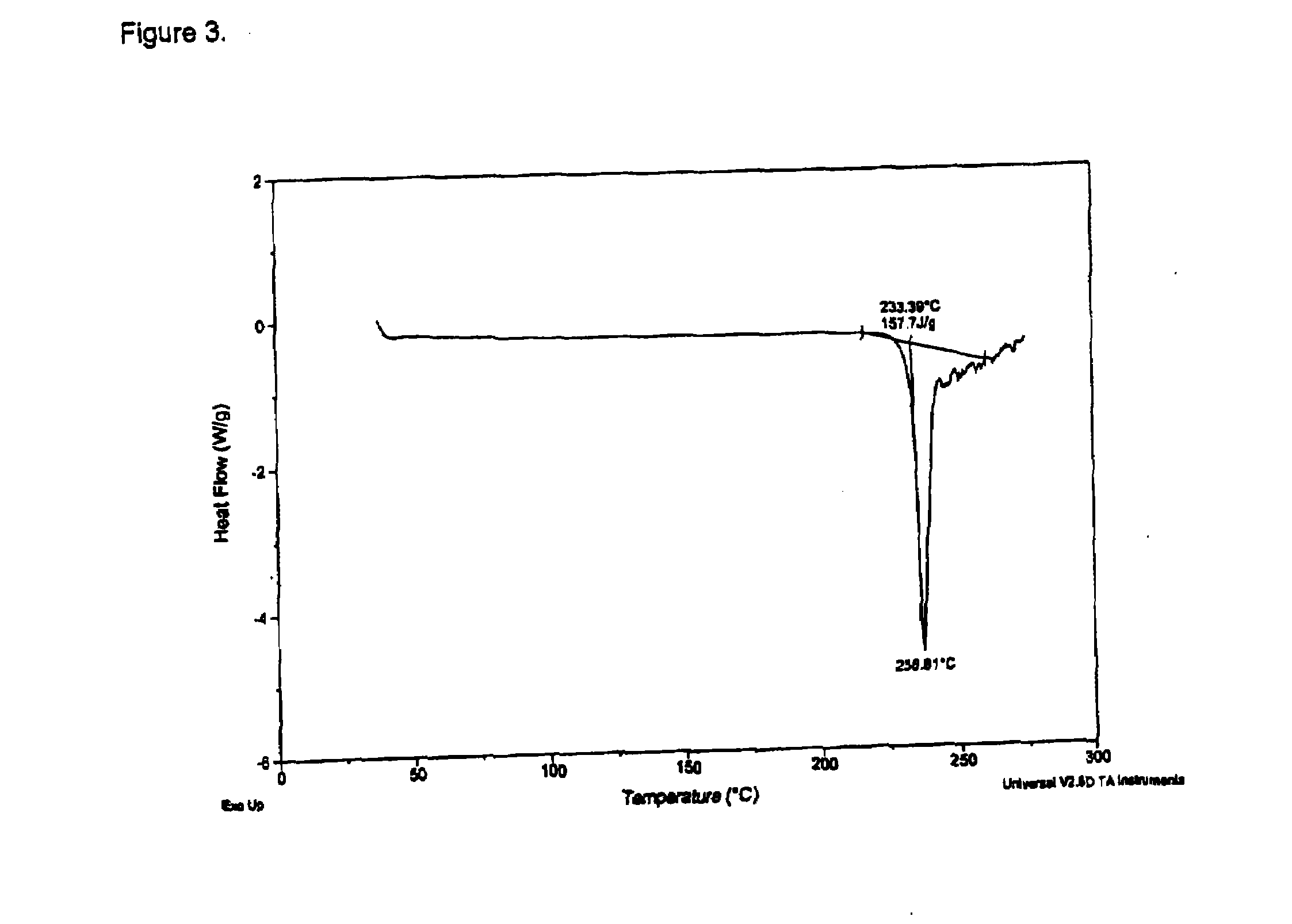
[0152]Ephedrine Hydrochloride (6.1 g) was stirred in USP water (45.0 g) to yield a solution pH of about 4.9. In a separate flask a disodium pamoate (6.5 g) solution was prepared using USP water (54.0 g) to yield a solution pH of about 9.5. The ephedrine HCl solution was transferred to a metered addition funnel and added to the disodium pamoate solution over approximately 1 h. When about one-half of the addition was completed, the solution became opaque. USP water (2.6 g) was used to rinse in the residue from the addition funnel. The opaque mixture was heated from about 26° C. to near 80° C. The reaction was stirred at about 80° C. for approximately 4.5 h. A thick residue settled in the reaction vessel as the solution cooled. The solution was decanted and the residue transferred to drying dishes. The residue was dried at about 100° C. under vacuum (nitrogen sweep) for about 5.5 h. Drying was continued for about 66 h, the solid ground with a mortar and ...
example 3
Alternate Preparation of Ephedrine Pamoate
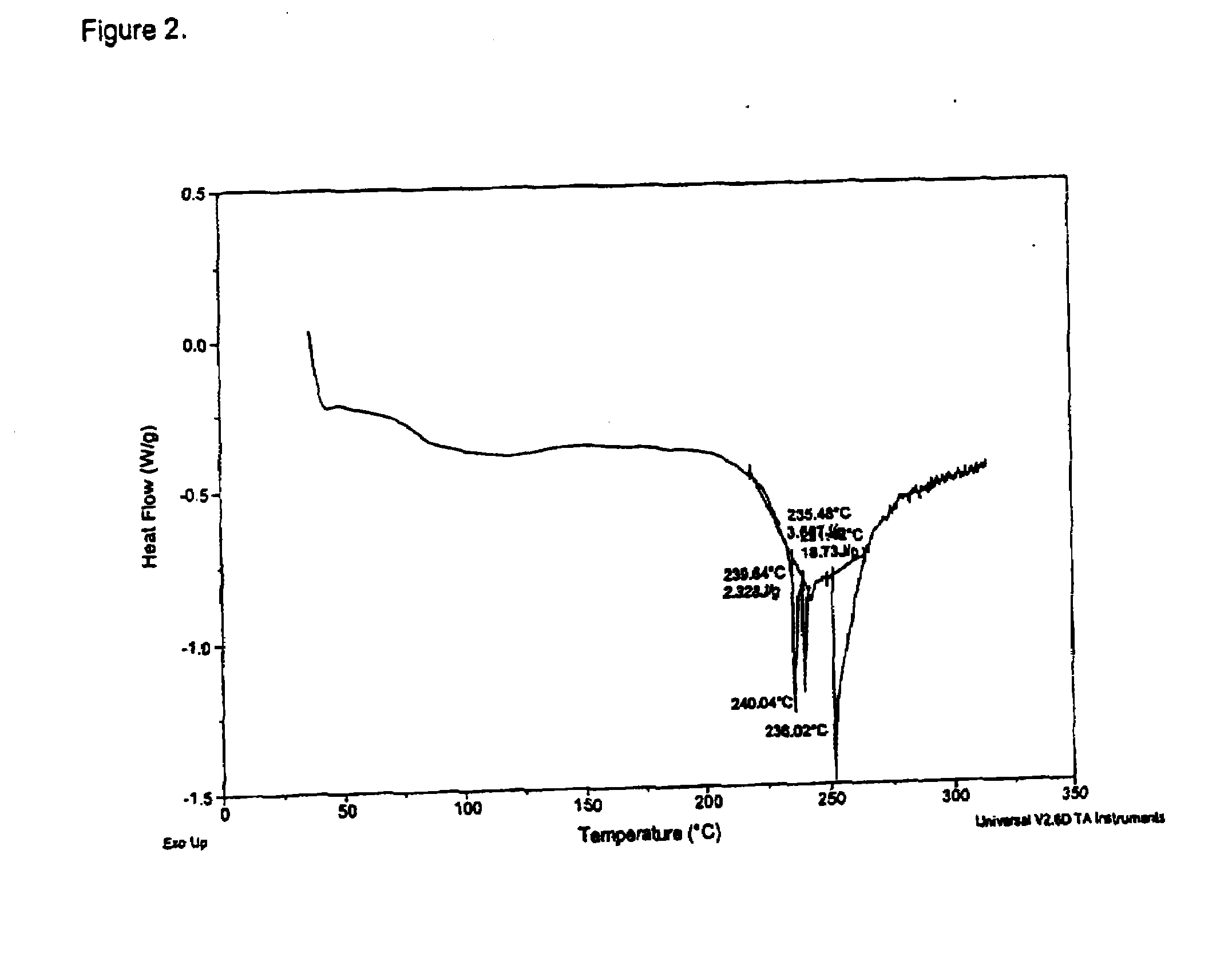
[0153]Ephedrine Hydrochloride (7.1 g) was stirred in methanol (62.6 g). Disodium pamoate (8.0 g) was stirred in USP water (71.0 g) and adjusted to about pH 9.5. The methanolic ephedrine hydrochloride solution was added to the disodium pamoate solution over a period of about 1.75 h. The reaction solution was heated to about 60° C. for around 4.5 h then cooled. The reactor was equipped with a distillation head and aqueous methanol was removed by heating. The opaque mixture was cooled and a residue settled to the bottom of the reaction vessel. The solution was carefully decanted and the residue was transferred to a drying dish. The material was placed in a vacuum drying oven (103° C.) for 24 h with a nitrogen sweep. Ephedrine Pamoate was obtained as a solid, 8.4 g (66.6%) 2:1 ephedrine pamoate (by HPLC assay) and characterized by DSC (FIG. 2), FTIR (FIG. 5) and PXRD (FIG. 8).
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com