Compositions and Methods for Therapy and Diagnosis of Cancer and Cancer Metastasis
a technology for cancer and metastasis, applied in the field of compositions and methods for cancer and cancer metastasis, can solve the problems of analyzing the resected primary tumor to predict the risk of metastasis, it is difficult if not impossible to predict whether a primary tumor has metastasized, and cancer is still among the leading causes of death. it reduces the signaling of constitutive pip3
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0203]Tissues and Cell Lines
[0204]This study was approved by the local ethical review board (“Ethikkommission der Ärztekammer des Landes Rheinland-Pfalz”). Recombinant DNA work was done with the official permission and according to the rules of the state government of Rheinland-Pfalz. Tissues were obtained as human surplus materials during routine diagnostic or therapeutic procedures and were stored at −80° C. until use. If not otherwise stated, cell lines were obtained from commercial providers. For demethylation studies cells were split to 20-30% confluency and cultured with 2 μM or 10 μM 5-Aza-2′-deoxycytidine (5-Aza-dC) (Sigma-Aldrich) for 72 h. Colon cancer cell lines HCT116WT, HCT116DNMT1− / −, HCT116DNMT3b− / − and HCT116DKO were kindly provided by Bert Vogelstein.
[0205]RNA-Isolation, RT-PCR and Real-Time RT-PCR
[0206]RNA extraction, first-strand cDNA synthesis, RT-PCR and real-time RT-PCR were performed as previously described (Koslowski, M. et al., Cancer Re...
example 2
TPTE is Ectopically Expressed in Human Tumors
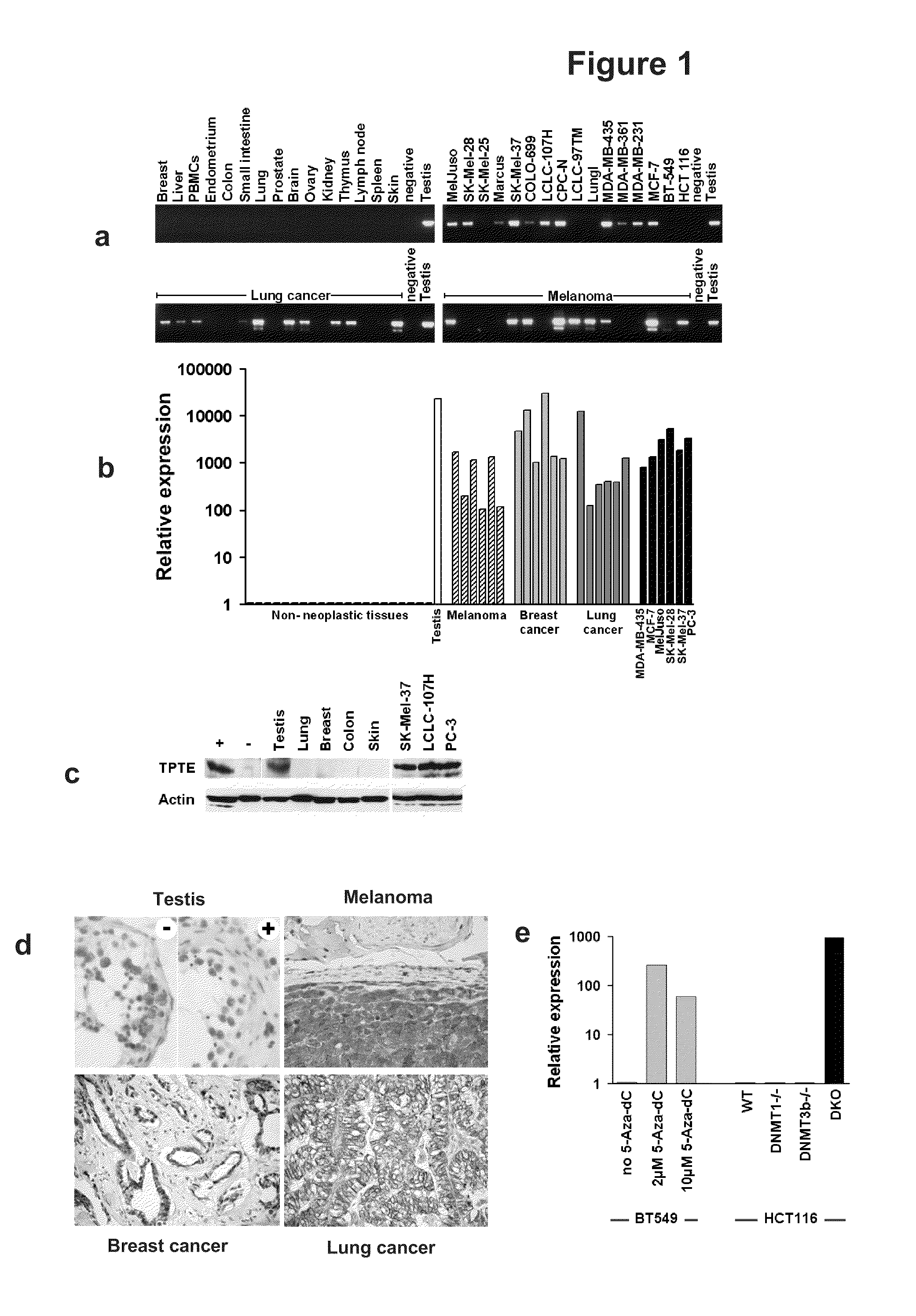
[0230]TPTE mRNA expression was investigated in a large set of normal and neoplastic tissue specimens. TPTE expression is confined to testis and transcript amounts are below detection limit of highly sensitive RT-PCR in all other normal tissue specimens (FIGS. 1a,b). In contrast, strong TPTE expression was detected in 59 of 155 (38%) tumor samples across different cancer types including malignant melanoma (50%), breast carcinomas (47%) and lung carcinomas (55%) as well as in a large set of cancer cell lines (62%) (Tab. 1).
TABLE 1Expression of TPTE in human tissues and cell lines analyzed byRT-PCR and Real-Time PCR.Positive / testedNormal tissuesTestis3 / 3Small intestine0 / 2Colon0 / 3Liver0 / 2Lung0 / 3Lymph node0 / 2Stomach0 / 2Spleen0 / 2Adrenal gland0 / 1Kidney0 / 3Esophagus0 / 1Ovary0 / 2Thymus0 / 1Skin0 / 2Breast0 / 3Pancreas0 / 2PBMC's, resting0 / 3PBMC's, proliferating0 / 3Prostate0 / 2Thyroid0 / 2Endometrium0 / 3Cerebellum0 / 1Brain0 / 2Tumour tissuesBreast cancer17 / 36 (47%)Lun...
example 3
TPTE is a Plasma Membrane PIP3-Phosphatase
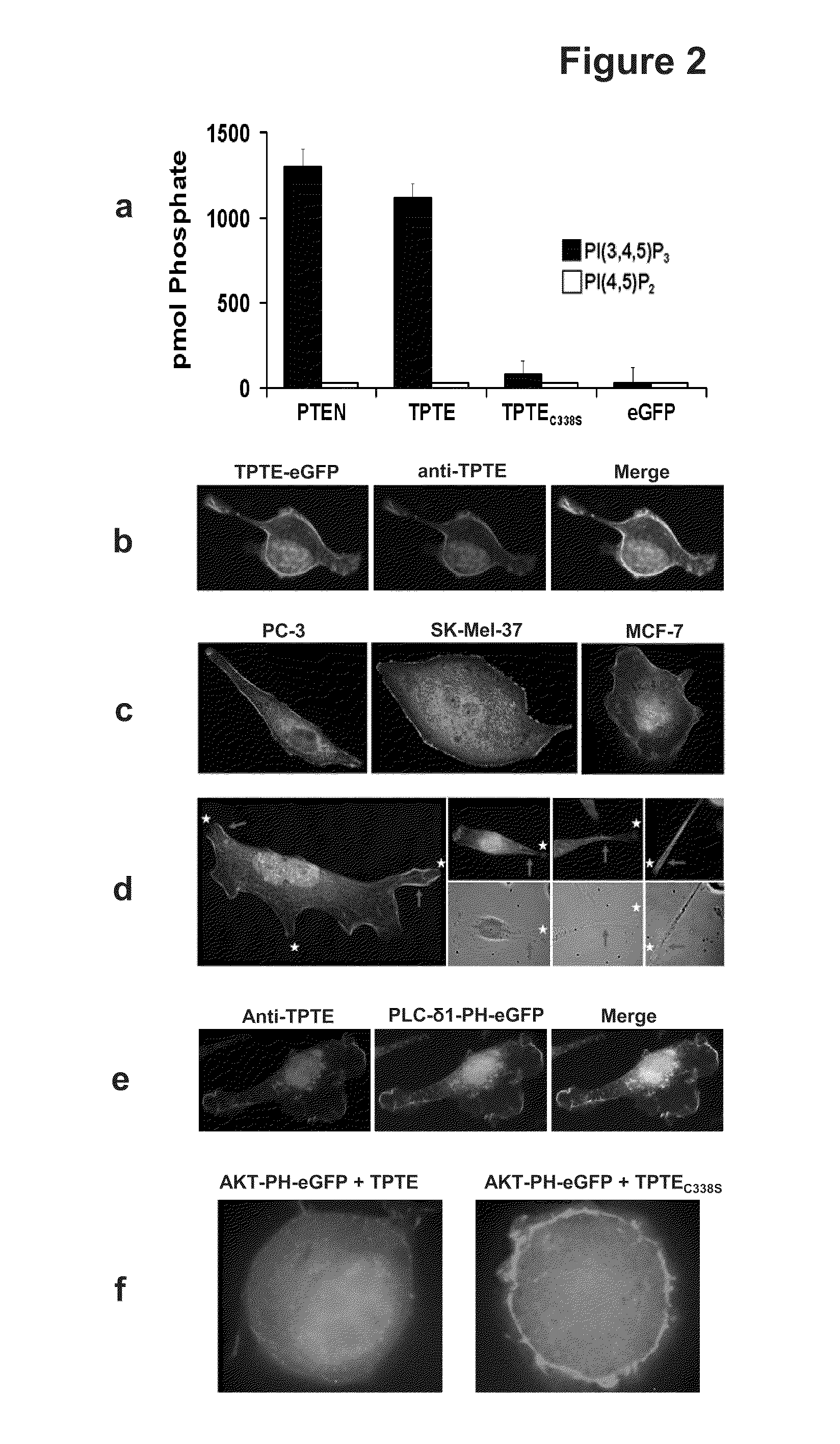
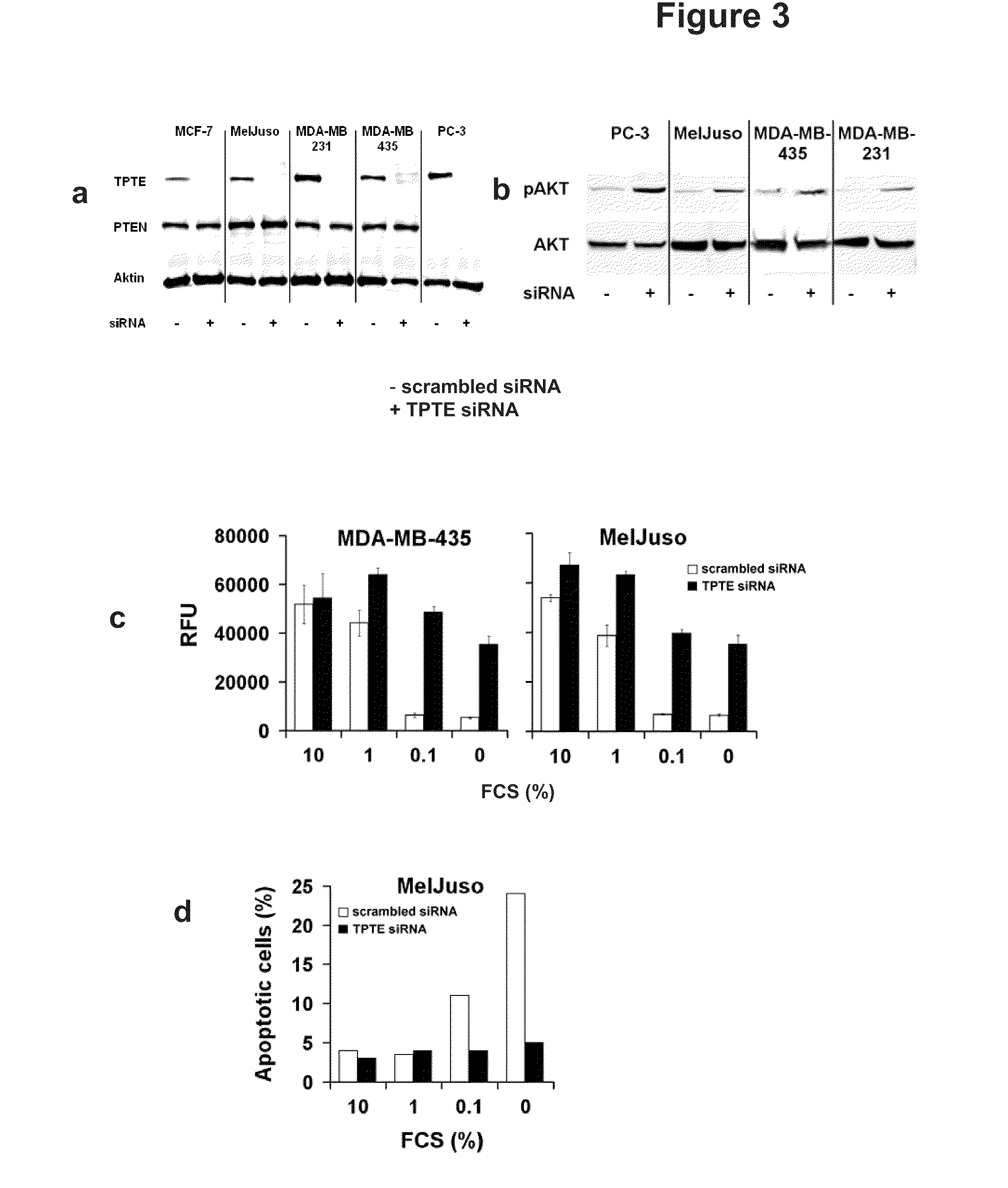
[0234]TPTE contains a phosphatase as well as a lipid-binding C2 domain, which have been shown to be essential and sufficient for the lipid phosphatase activity of its homologue PTEN (Lee, J. O. et al., Cell 99, 323-334 (1999)). Whereas a lipid phosphatase activity with substrate-specificity for PIP3 and PI(3,4)P2 has previously been shown for the mouse orthologue of TPTE (Wu, Y. et al., J. Biol. Chem. 276, 21745-21753 (2001)) in vitro, no enzymatic activity was detected for the human counterpart (Walker, S. M. et al., Biochem. J. 360, 277-283 (2001)), leaving PTEN as the only so far known human PIP3-phosphatase. Since the latter study used recombinant protein of bacterial origin, enzymatic activity of human TPTE with eucaryotically produced protein was reassessed. The phosphatase and C2 domains of TPTE and PTEN fused to eGFP were expressed in HEK-293 cells, the proteins purified by immunoprecipitation with anti-eGFP antibody coupled protein ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com