Compositions and methods for performing reverse gene therapy
a reverse gene therapy and compound technology, applied in the field of gene therapy adaptation, can solve the problems of harmful mutations, less effective expression of host cells, and inability to provide therapeutic benefits of vectors, and achieve the effect of reducing disease or disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Ibutilide Controlled Release Matrices for Preventing Re-Entrant Atrial Flutter in Dogs
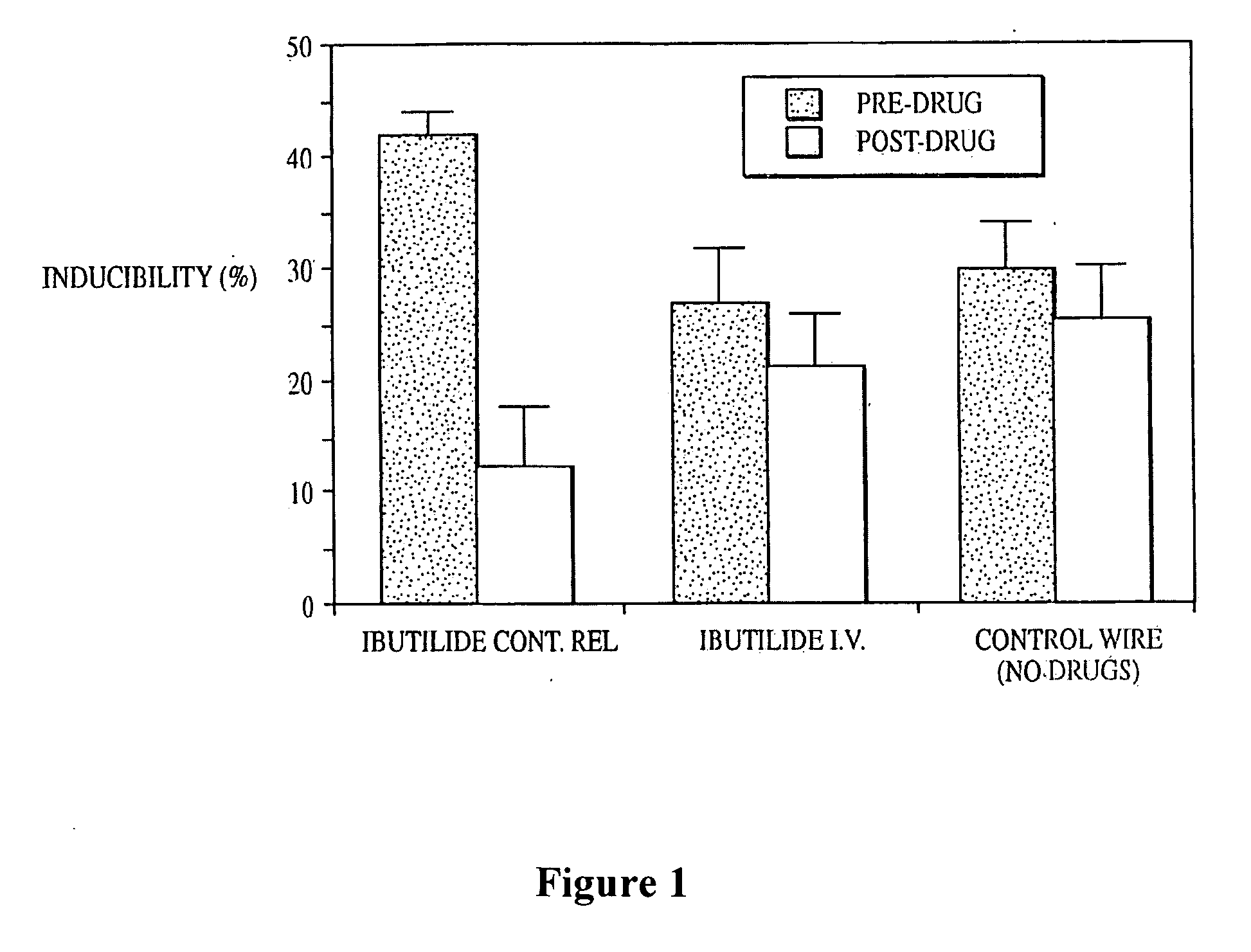
[0164]In the experiments described in this Example, the Y-atriotomy model for re-entrant flutter in dogs, as described (Labhasetwar et al., 1998, J. Cardiovasc. Pharmacol. 31:449-455) was used to demonstrate the efficacy of sustained release of ibutilide from a right atrial epicardial implant for alleviating re-entrant atrial flutter.
[0165]Ibutilide sustained release matrices were made using a multi-layer polyurethane solvent evaporation technique to coat an epicardial pacing electrode. Inducibility of atrial flutter upon burst atrial pacing was investigated in dogs which had a coated electrode implanted therein, compared with dogs which had a non-coated electrode implanted therein. As indicated in FIG. 1, inducibility of atrial flutter was significantly reduced in dogs which had a coated electrode implanted therein (“Ibutilide Cont. Rel” in FIG. 1). The rate of release of ibutilide from the electr...
example 2
HERG Gene Therapy of Re-Entrant Atrial Flutter in a Dog Model
[0167]The experiments described in this Example demonstrate that DNA-containing biodegradable polymeric microparticles and nanoparticles are useful for delivery of nucleic acid vectors to animal cells.
[0168]A reverse gene therapy method is used to locally deliver a nucleic acid vector comprising a defective HERG protein to the right atrium of dogs in order to effect site specific overexpression of HERG (A561V) at that site.
[0169]The nucleic acid vector is delivered in the form of a plasmid suspended in nanoparticles of a polylactic-polyglycolic acid (PLGA) copolymer having poly-L-lysine (PLL) incorporated therein. The plasmid DNA is in a condensed form. Prior to using the nucleic acid vector encoding defective HERG, a reporter vector comprising a nucleic acid encoding a bacterial β-galactosidase or a luciferase operably linked with a CMV promoter is used to assess the level and localization of expression effected by PLGA / P...
example 3
Gene Therapy Using a Cardiac Myocyte Model
[0184]The Experiments described in this Example may be used to demonstrate that a nucleic acid vector comprising an expression vector encoding the HERG (A561V) protein may be delivered to atrial myocardium cells in order to alleviate re-entrant atrial flutter.
CHO Cell Transformation Studies
[0185]Transformation of Chinese Hamster Ovary (CHO) cells in vitro is used to investigate the mechanism(s) by which the cells are transformed using DNA-PLGA-PLL nanoparticles. Transformation of CHO cells is also used to investigate the effects of nanoparticle formulation parameters (e.g. the effect of including or omitting PLL from the particles) on the steps involved in nanoparticle uptake, endosomal or lysosomal transit of the nanoparticles within the cells, and nuclear expression of vector DNA. Properties of transformed CHO cells which are assessed include, but are not limited to, histological or immunological examination of the location of vector DNA e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltage | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com