Resveratrol Ferulate Compounds, Compositions Containing The Compounds, And Methods Of Using The Same
a technology of ferulate and resveratrol, which is applied in the field of new chemical compounds as well as cosmetic or pharmaceutical compositions, can solve the problems of general instability of cosmetic formulations, reduced melanin total amount in the skin, and less desirable, and achieve the effect of stable cosmetic compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Resveratrol Ferulates by Liquid-Phase Esterification
[0186]91.3 grams of resveratrol (approximately 0.4 M) was first dissolved in 300 ml of tetrahydrofuran (THF) to form a first solution. 77.7 grams of ferulic acid (approximately 0.4 M) was dissolved in 300 ml of THF to form a second solution. 0.1 gram of p-toluene sulfuric acid was dissolved in 20 ml of THF to form a third solution. The first, second, and third solutions were then transferred into a 1000 ml round-bottom flask equipped with a condenser, followed by addition of 10 ml of benzene. The liquid mixture was heated until boiling, and the boiling was continued under reflux for 5 hours to collect 50 ml of distillate. Next, 50 ml of THF and 10 ml of benzene were added into the liquid mixture, which was continued to be heated under reflux for another 5 hours to collect another 50 ml of distillate. Another 50 ml of THF and 10 ml of benzene were added into the liquid mixture, followed by continuous heating of the liqu...
example 2
Antioxidant Activity of Resveratrol Ferulates
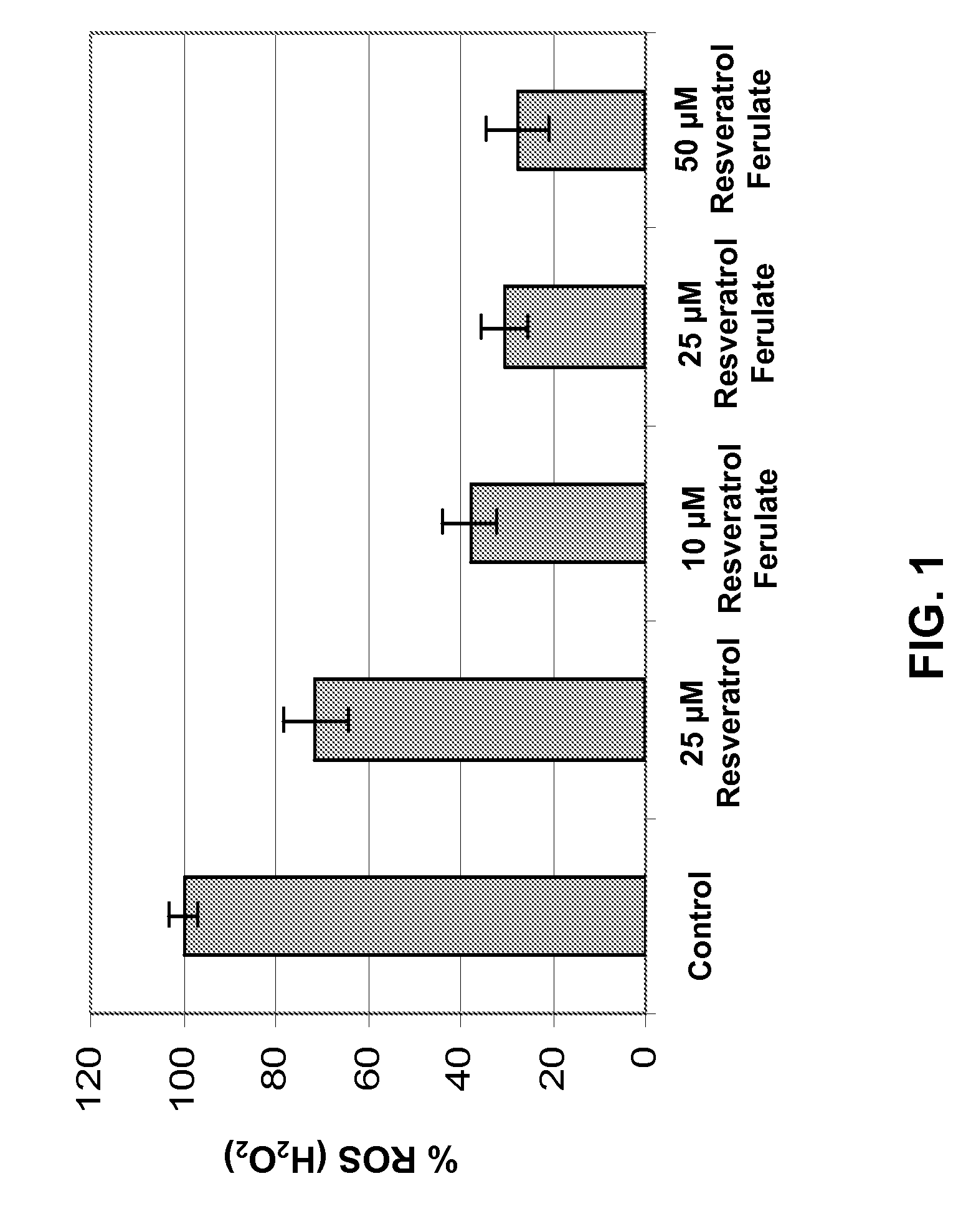
[0190]Resveratrol Ferulates of the present invention demonstrated surprising and expected efficacy in reducing endogenous reactive oxygen species (ROS), primarily hydrogen peroxide (H2O2), in normal human epidermal keratinocyte (NHEK) cell cultures.
[0191]Specifically, NHEK cells were cultured and plated, followed by treatment with Resveratrol Ferulates at concentrations of about 10 μM, 25 μM, and 50 μM. As comparative examples, some NHEK cells were separated treated with resveratrol at a concentration of about 25 μM. Further as control examples, some NHEK cells were maintained without treatment with either Resveratrol Ferulates or resveratrol. Four samples of NHEK cells were provided for each treatment. After overnight incubation of the cell cultures, dichlorodihydrofluorescein diacetate was added thereinto. Dichlorodihydrofluorescein diacetate is a useful fluorogenic reagent for detecting reactive oxygen species in cells. Upon oxidation ...
example 3
Non-Cytotoxicity of Resveratrol Ferulates
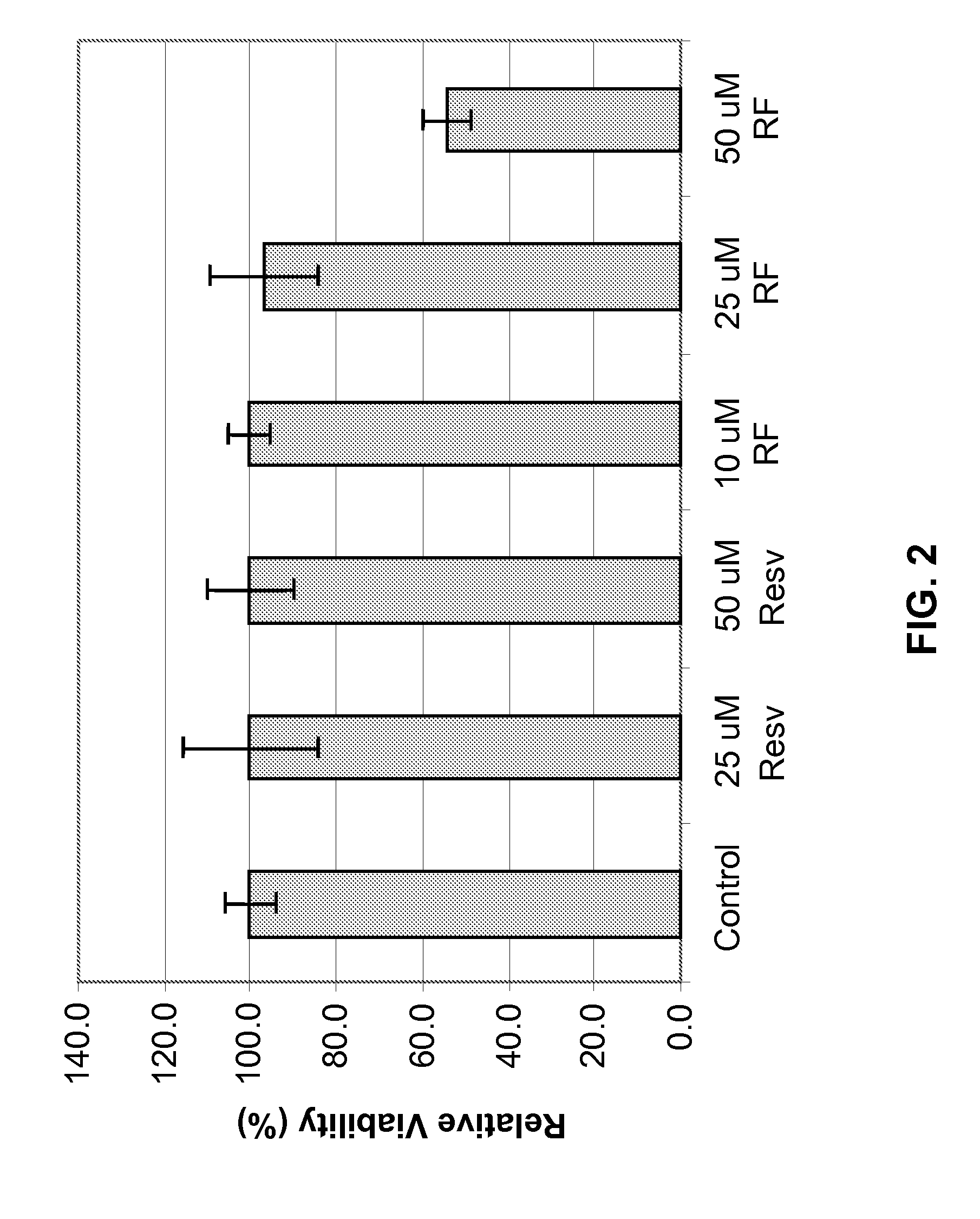
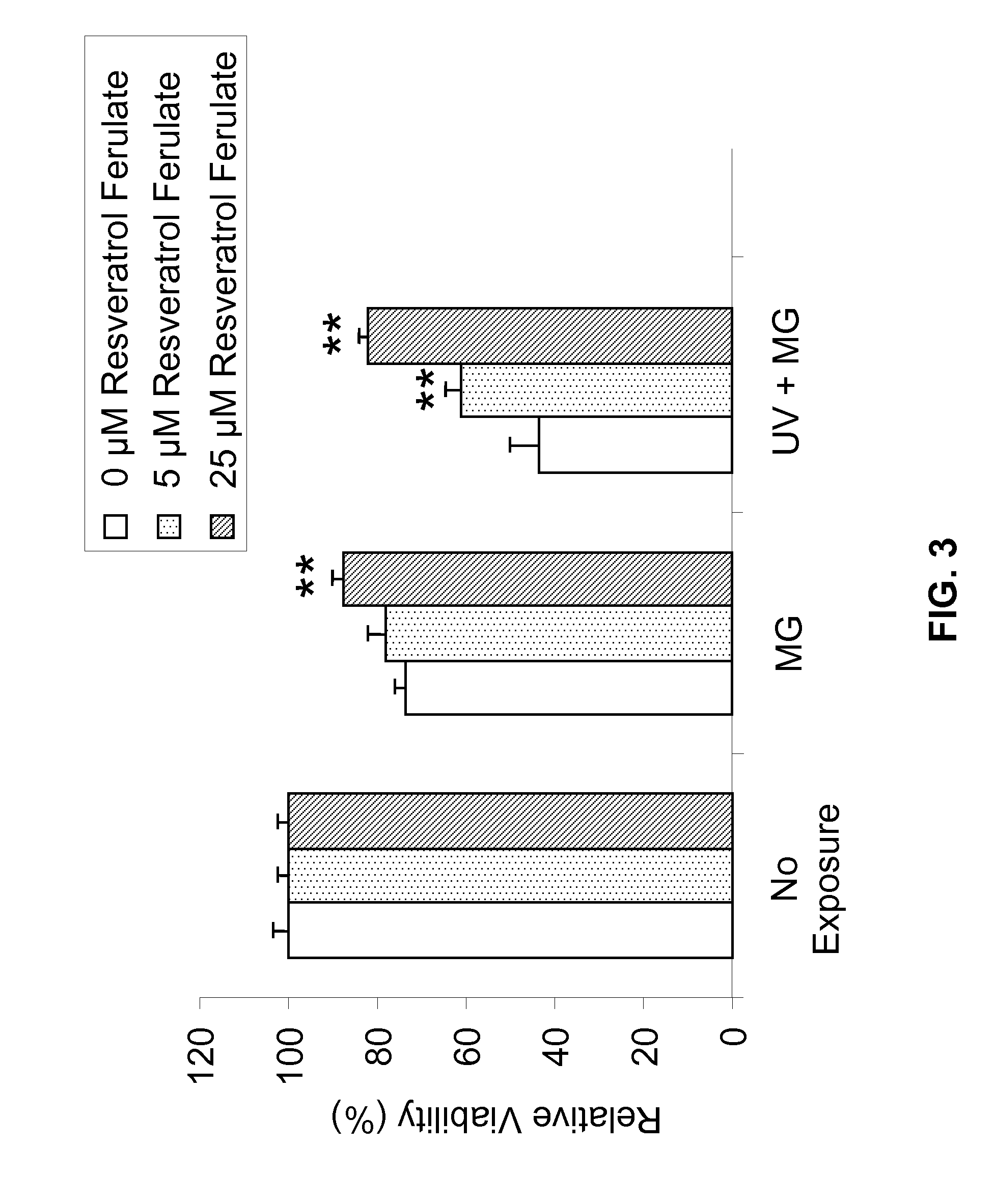
[0193]Metabolism in viable cells produces “reducing equivalents” such as NADH or NADPH. These reducing compounds pass their electrons to an intermediate electron transfer reagent that can reduce a tetrazolium product, MTS [(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfopheny-1)-2H-tetrazolium], into an aqueous, soluble formazan product. At death, cells rapidly lose the ability to reduce tetrazolium products. The production of the colored formazan product, therefore, is proportional to the number of viable cells in culture. In other words, light absorbance by formazan produced in the cell culture can be used as an indicator of the cell viability.
[0194]Specifically, NHEK cells were cultured and plated, followed by treatment with Resveratrol Ferulates at concentrations of about 10 μM, 25 μM, and 50 μM. As comparative examples, some NHEK cells were separated treated with resveratrol at concentrations of about 25 μM and 50 μM. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com