Non-basic melanin concentrating hormone receptor-1 antagonists and methods
a hormone receptor and non-basic melanin technology, applied in the direction of drug compositions, peptide/protein ingredients, metabolic disorders, etc., can solve the problems of increased food intake, weight loss, and decreased feeding in diet-induced obese mi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific embodiments
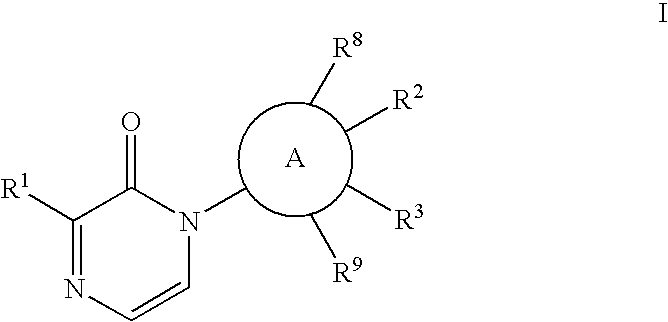
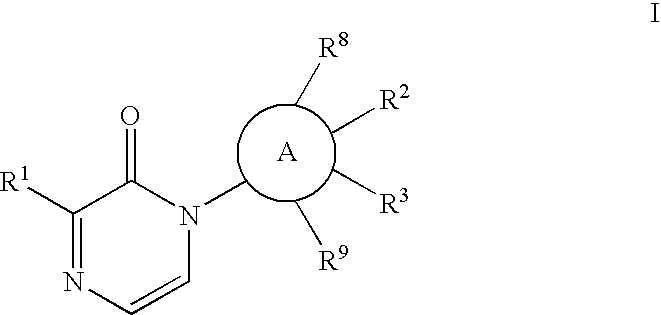
[0051]The
group may be phenylene or a heteroaryl which is monocyclic or bicyclic and includes rings such as
[0052]The Formula I compound of the invention may have the structure
[0053]In the compound of Formula I of the invention, it is desired that
is phenylene or pyridinyl;
[0054]X is O or S; and / or
[0055]Y is a bond or an alkylene chain of 1, 2 or 3 atoms; and / or
[0056]Z is phenyl; or
[0057]Z is a heteroaryl such as pyridinyl or benzothiazole; and / or
[0058]R2 is E-G-J; and / or
[0059]E is O or S; and / or
[0060]G is a lower alkyl or alkylcycloalkyl; and / or
[0061]J is H, OH, SO2R7, lower alkyl, lower alkoxy, or CF3, more preferably OH; and / or
[0062]R3 is C1-6 alkyl, C1-6 alkoxy, H, or halo; and / or
[0063]R8 and R9 are independently H or CH3; and / or
[0064]wherein R2 and R3 can be taken together to form a 5- to 7-membered ring which is saturated, unsaturated, or partially unsaturated and may include an E heteroatom, which is O, or 0, 1 or 2 N atoms, which ring is substituted with one or two of —O-G-(J)m...
example 193
1-(2-Methoxy-4-(2-oxo-3-(4-(trifluoromethyl)phenylthio)pyrazin-1(2H)-yl)phenoxy)-2-methylpropan-2-yl 2-aminoacetate
[0304]
Part A. 1-(2-Methoxy-4-(2-oxo-3-(4-(trifluoromethyl)phenylthio)pyrazin-1(2H)-yl)phenoxy)-2-methylpropan-2-yl 2-(tert-butoxycarbonylamino)acetate
[0305]
[0306]To a stirred suspension of the alcohol prepared in Example 36 (3.0 g, 6.4 mmol), 4-pyrrolidinopyridine (0.95 g, 6.4 mmol) and BOC-glycine (3.4 g, 19 mmol) in CH2Cl2 (60 mL) at 42° C. was added N,N′-diisopropylcarbodiimide 3.0 mL, 19 mmol) over 3.5 h. After stirring at reflux for 2.5 h, HPLC analysis showed 25% alcohol still remained. More BOC-glycine (3.4 g, 19 mmol) was added followed by additional N,N′-diisopropylcarbodiimide (3.0 mL, 19 mmol) which was slowly added over 3.5 h; whereupon, HPLC analysis showed 3 (3×20 mL) prior to drying over MgSO4 and concentrating under vacuum to afford 5.6 g of crude product. Chromatography (silica gel 230-400 mesh, gradient elution: 0 to 60% EtOAc / hexane over 47 min) of th...
examples 194 to 201
[0309]Examples 194 to 201 were prepared in a similar manner to Example 193 using the appropriate alcohol and BOC glycine followed by TFA removal of the BOC group.
TABLE 4Glycine Prodrug EstersGlycine ester ofHPLC1H-NMRExample No.Example No.(Met1)LC MS(CDCl3)194383.865407.55 (d, J = 9.3 Hz, 2H), 7.27 (d, J = 9.3 Hz,2H), 7.18 (d, J = 4.4 Hz, 1H), 7.06 (d, J = 4.4 Hz,1H), 7.01 (d, J = 2.2 Hz, 1H),6.99 (d, J = 8.2 Hz, 1H), 6.86 (dd, J = 2.2 and 8.2 Hz,1H), 4.15 (s, 2H), 3.76 (s, 3H), 3.17 (s,2H), 1.50 (s, 6H).195323.594707.47 (d, J = 8.7 Hz, 2H), 7.27 (d, J = 8.7 Hz,2H), 7.11 (d, J = 4.4 Hz, 1H), 6.98 (d, J = 2.3 Hz,1H), 6.97 (d, J = 8.5 Hz, 1H),6.95 (d, J = 4.4 Hz, 1H), 6.88 (dd, J = 2.3 and8.5 Hz, 1H), 4.20 (s, 2H), 3.86 (s, 3H),3.33 (s, 2H), 2.40 (s, 3H), 1.50 (s, 6H).196503.784847.34 (d, J = 8.3 Hz, 2H), 7.22 (d, J = 8.3 Hz,2H), 7.05 (d, J = 4.4 Hz, 1H), 6.92 (d, J = 2.2 Hz,1H), 6.90 (d, J = 8.2 Hz, 1H),6.87 (d, J = 4.4 Hz, 1H), 6.83 (dd, J = 2.2 and8.2 Hz, 1H), 4.14 (s, 2H), 3.79 (s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com