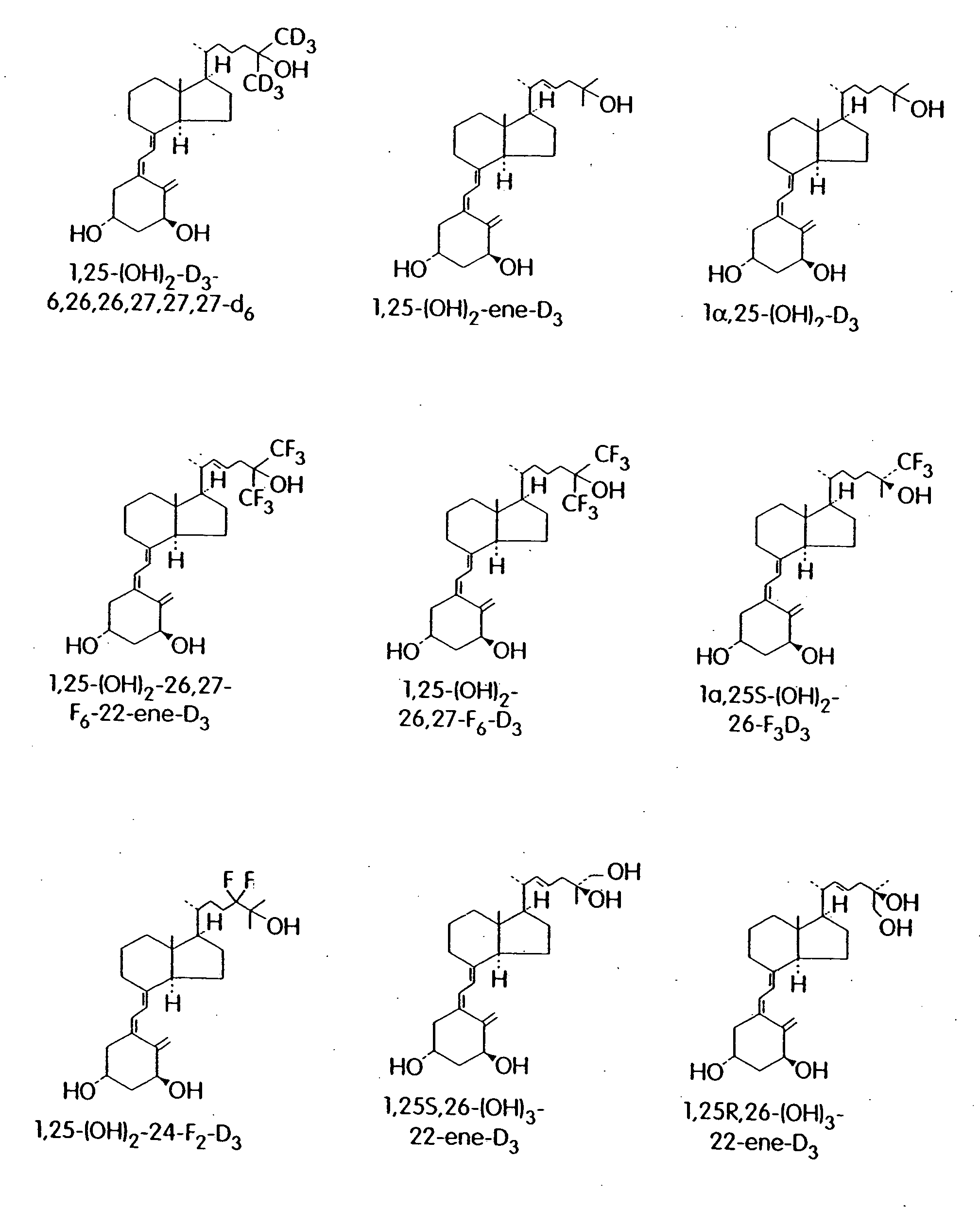
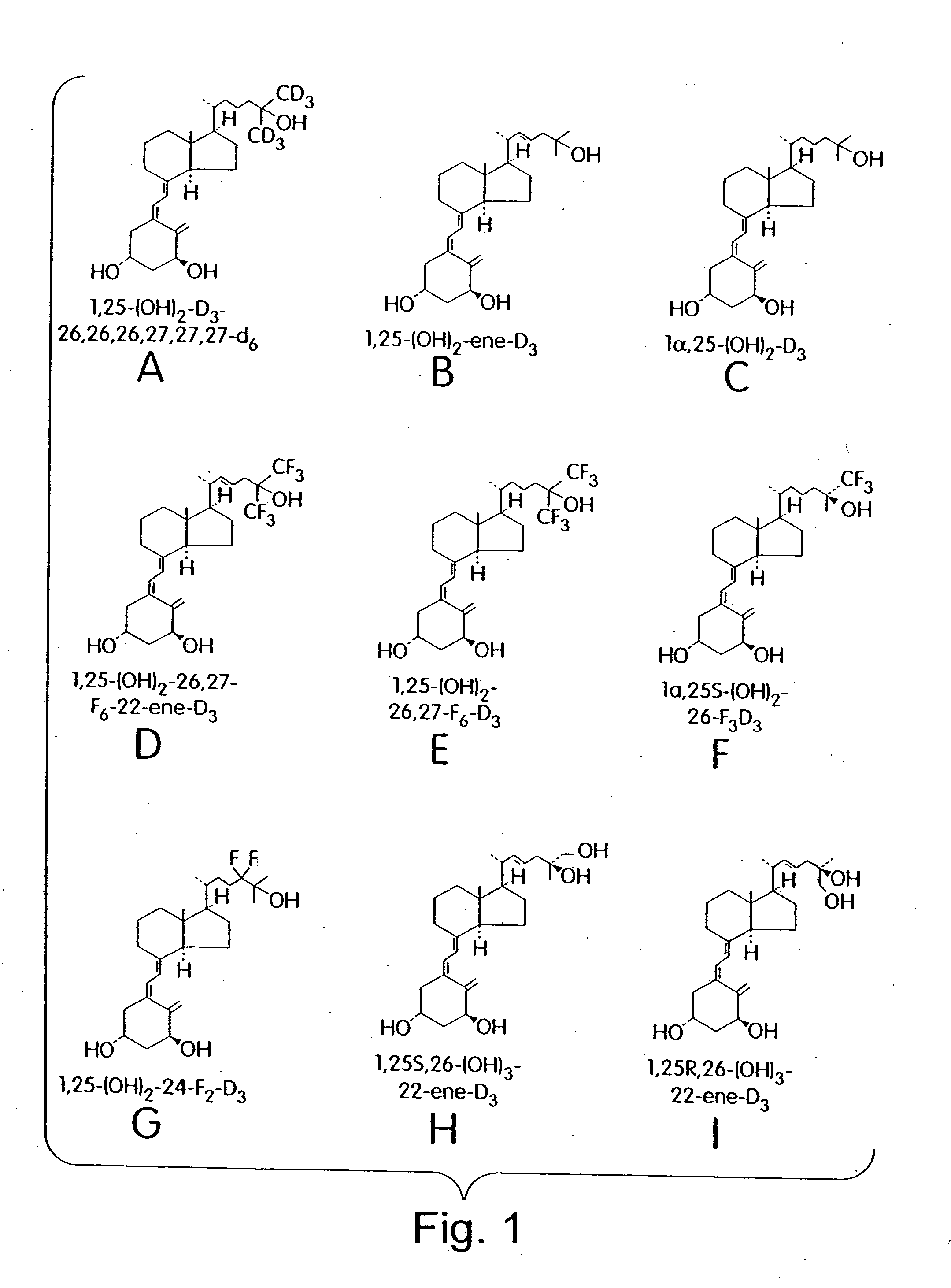
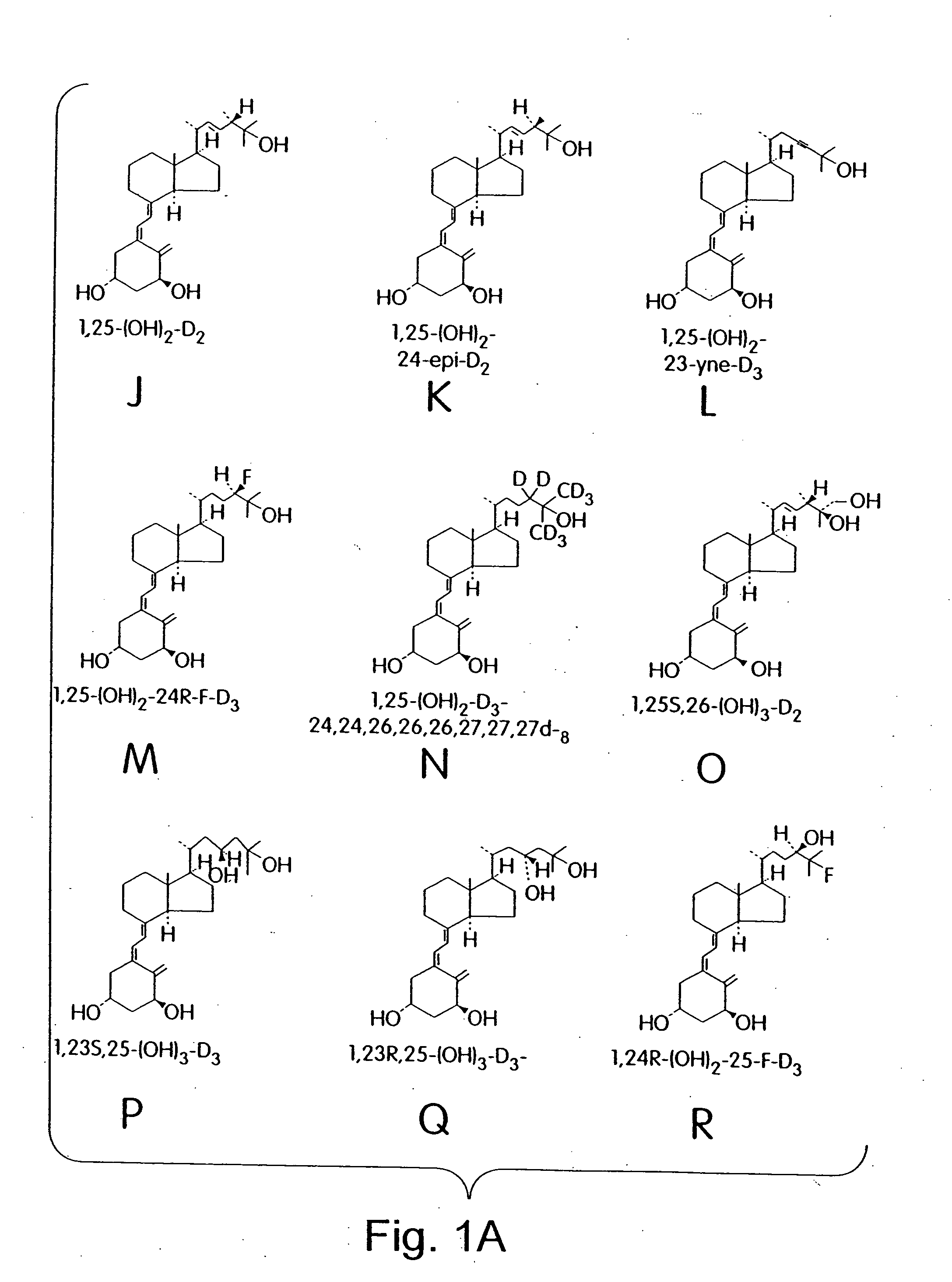
Cyclic ether vitamin D3 compounds, 1alpha(OH)3-epi-vitamin D3 compounds and uses thereof
a technology of cyclic ether and vitamin d3, which is applied in the field of cyclic ether vitamin d3 compounds, 1alpha (oh) 3epivitamin d3 compounds, can solve the problems of limited clinical applications of vitamin d/sub>3 and its structural analogs, and achieves enhanced stability, reduced growth or activity of cells, and improved biological properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation and Identification of a Cyclic Ether Metabolite of 1α,25-dihydroxy-Vitamin D3 in Human Keratinocytes
[0180]As described herein, 1α25(OH)2-3-epi-D3 is metabolized into a less polar metabolite than 1α25(OH)2-3-epi-D3, peak M1, in human keratinocytes (FIG. 2). FIG. 2 shows the HPLC profile and UV spectra of the metabolites produced in human keratinocytes incubated with 1α25(OH)2-3-epi3D3 (1 uM) for 24H. On a straight phase HPLC system, this metabolite (M1) is more polar than 25(OH) D3 but less polar than 1α25(OH)2D3 and similar to that of 1α(OH)D3. Mass spectrometric analysis reveals a molecular ion of 414 m / z, which is 2 mass units less than the starting 1α25(OH)2D3, shown in FIG. 3. FIG. 3 shows the mass spectra of 1α(OH)2-3-epi-D3 (M) (upper panel) and its cyclic ether metabolite (M1) (lower panel) isolated from human keratinocytes incubated with 1α25(OH)2-3-epi-D3(1 uM) for 24 h. The typical fragments at m / z 134 and 152 m / z indicate an unmodified A-ring and cistriene struc...
example ii
Isolation and Identification of a 3-Epi Metabolite of 1α hydroxy-Vitamin D3 in Human Keratinocytes
[0182]FIG. 5A shows the metabolism of 1α(OH)-D3 into its 3 epi form in the osteosarcoma cell line UMR-106. Peak A represents the 3-epi form of 1α(OH)D3. Peak B corresponds to the substrate, 1α(OH)D3. The insert panels show the UV spectra of the various metabolites as monitored by photodiode array detector. FIG. 5B shows a schematic representation of the 3-epimerization of 1α(OH)D3 into 1α(OH)-3-epi vitamin D3. Similar to 1α(OH)D3, 1α(OH)-3-epi vitamin D3 can be converted into the 25-hydroxylated form in vivo.
[0183]1α(OH)D3 compounds are currently used in the treatment of osteoporosis. Thus, 3-epi forms of these compounds may be used as substitutes for 1α(OH)D3 compounds in treating osteoporosis.
example iii
Confirmation of 3-epi Configuration of 1α(OH) 3-epi Vitamin D3
[0184]To confirm the production of 1α(OH) 3-epi vitamin D3 in bone cells the metabolites of 1α-3-epi-D3 produced by the osteosarcoma cell line (UMR-106) were analyzed by mass spectroscopy. FIG. 6 shows the mass spectra of 1α(OH)D3 (upper panel) and its 3-epi metabolite (lower panel). A comparison of these two mass spectra revealed difference in peaks observed only in the 3-epi metabolite, for example, fragments having molecular weights of approximately m / z 57, 217, 312 and 529 (lower panel). The mass spectrum of the 3-epi metabolite was independently confirmed to be 1α(OH) 3-epi vitamin D3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| chemical characterization | aaaaa | aaaaa |
| hydrophobic | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com