Immunomodulatory Peptides Derived from Heat Shock Proteins and Uses Thereof
a technology of immunomodulatory peptides and proteins, which is applied in the field of immunomodulatory peptides derived from heat shock proteins, can solve the problems of severe and in some cases generalized tissue damage, adverse effects, and decrease in patient's quality of life, and achieve the effects of reducing or inhibiting the inflammatory response, and modulating the immune response in the subj
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cellular Immune Response to Recombinant E. coli dnaJ
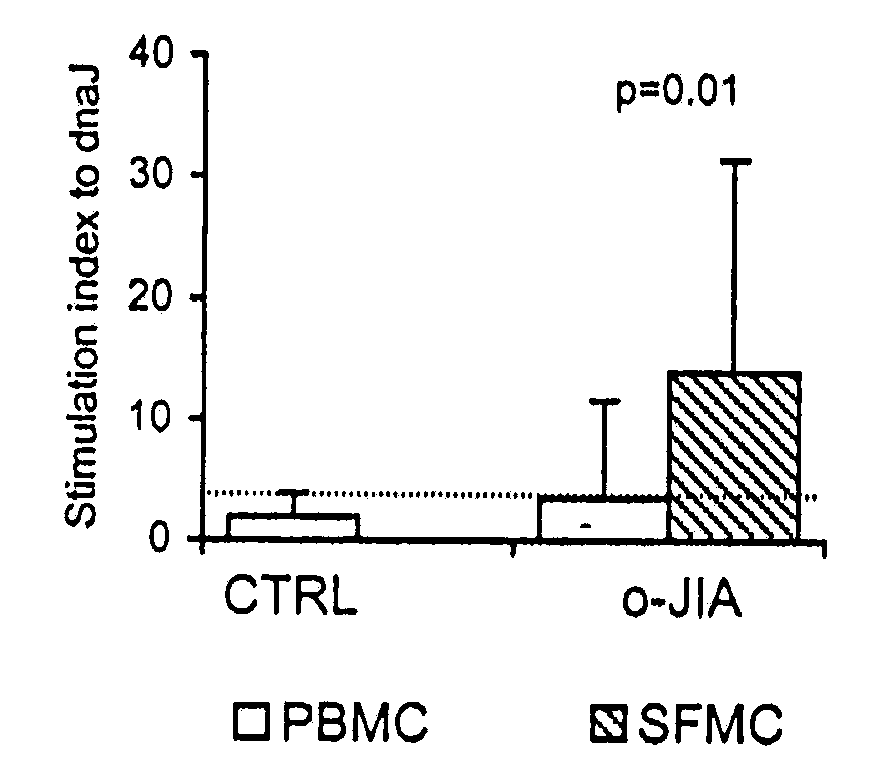
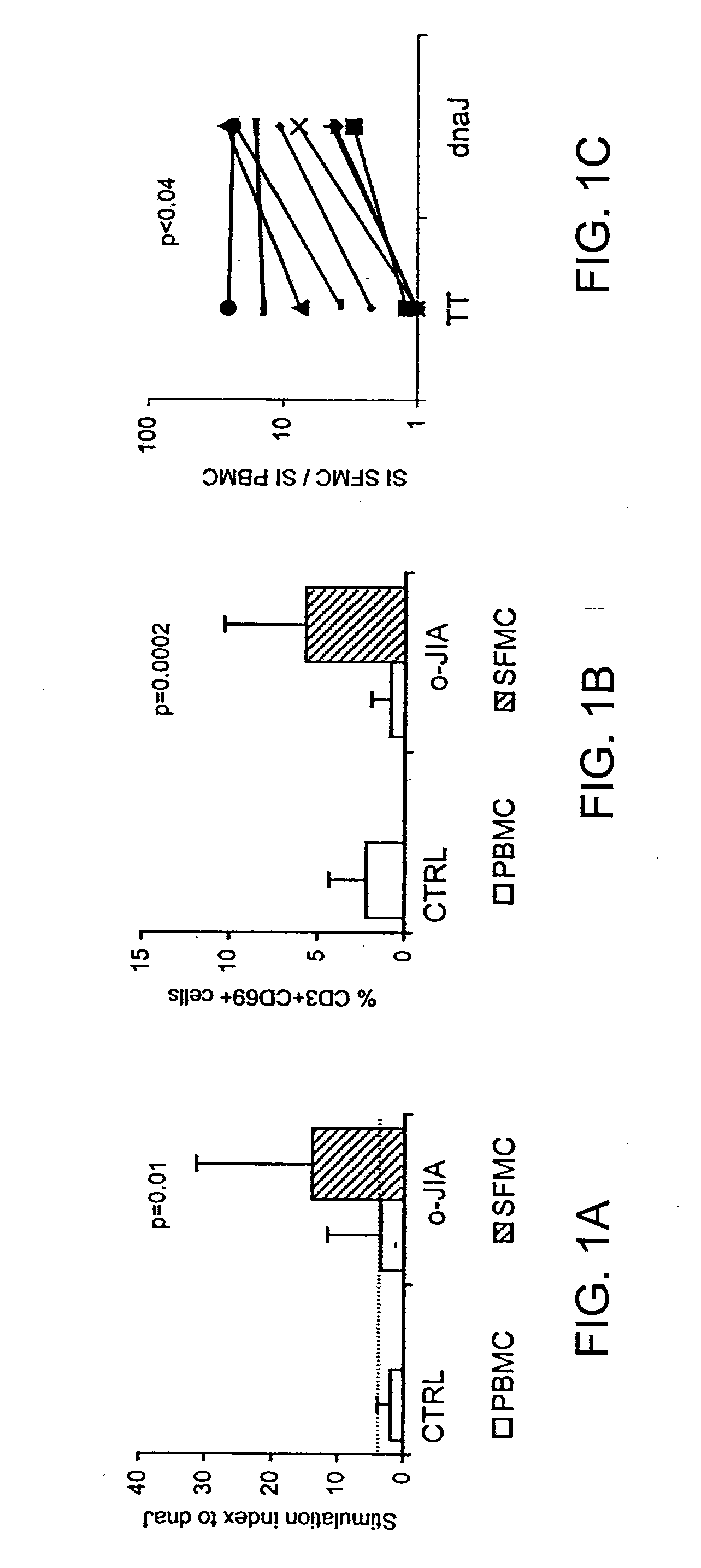
[0099]Proliferative responses to recombinant E. coli hsp dnaJ (dnaJ) of peripheral blood mononuclear cells (PBMC) from 10 healthy subjects (“control”) and 21 patients with oligoarticular juvenile idiopathic arthritis (“oJIA”) were not significantly different (FIG. 1A). To evaluate whether dnaJ-specific T cells were enriched at the synovial sites of inflammation, synovial fluid mononuclear cells (SFMC) from all patients with oJIA were stimulated with dnaJ for 96 hr. The mean stimulation index (SI) of SFMC was significantly higher than the SI of the corresponding PBMC (p=0.01; FIG. 1A). In addition, following stimulation with dnaJ, the percentage of activated T cells, evaluated as CD3+ CD69+ cells, was significantly higher (p=0.0002) in SFMC with respect to PBMC (FIG. 1B).
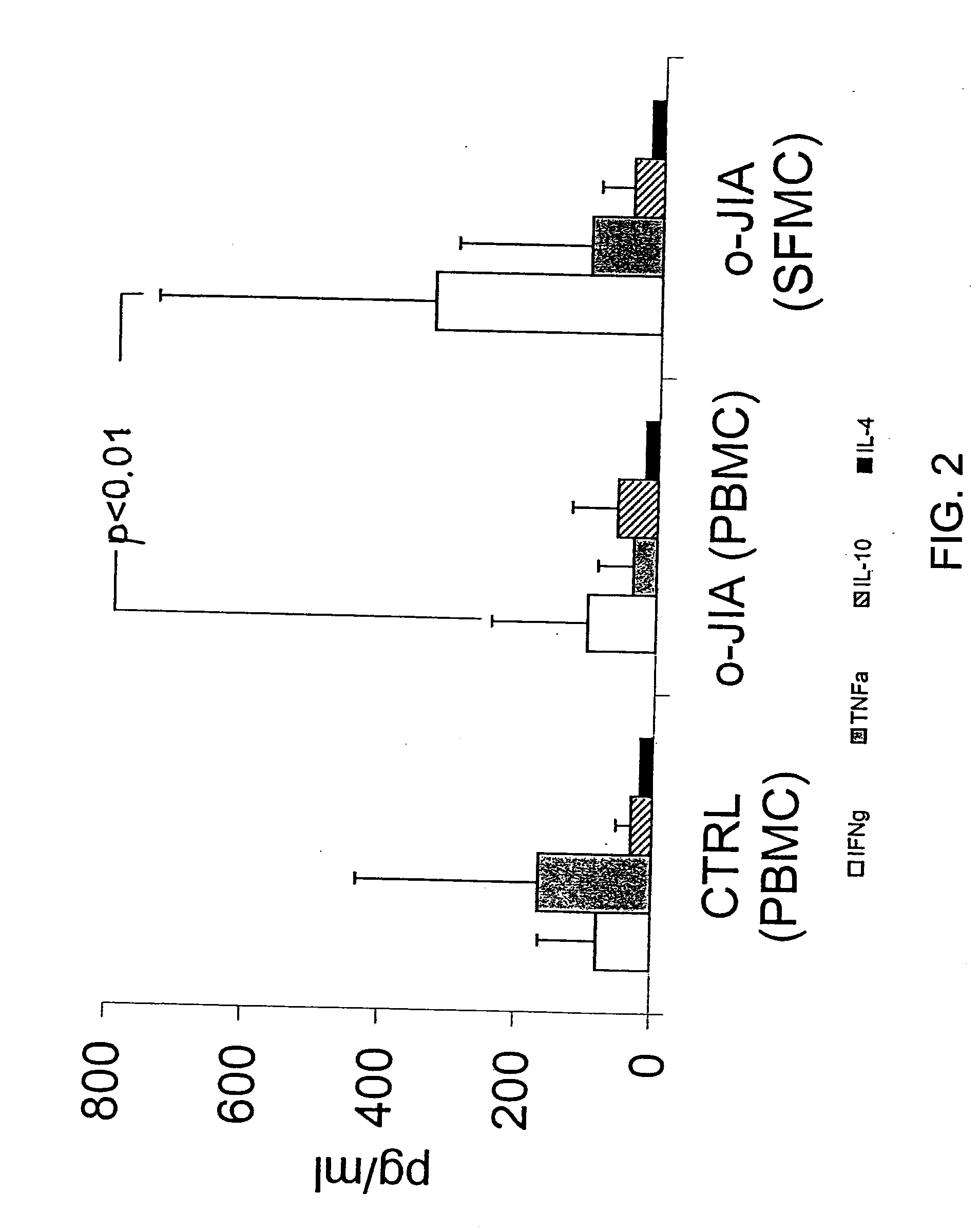
[0100]To confirm that the increased response of SFMC to dnaJ was not secondary to a non-specific increase in SFMC reactivity, the proliferative responses of cells f...
example 2
Cellular Immune Response to Synthetic Peptides Derived from Recombinant E. coli dnaJ
[0104]In order to identify possible relevant epitopes on the bacterial dnaJ protein, PBMC and SFMC of patients with oJIA were incubated for 72 hr with dnaJ, and restimulated with autologous feeder cells the in presence or absence of selected E. coli derived MHC class II binder peptides for 96 hr. A total of 8 peptides were tested, including three peptides (pep4, pep22, pep 174) that are derived from the N-terminal region of E. coli dnaJ and present sequence homologies to each of the three human dnaJ isoforms (HDJ1, HDJ2, HSJ1; homologous peptides), and five peptides that are derived from the C-terminal region of E. coli dnaJ and do not present any homology with the human counterparts (pep61, pep209, pep242, pep264, pep 268; non-homologous peptides.
[0105]The stimulation of PBMC with E. coli derived peptides resulted in very low proliferative responses (SI6 in the presence of peptide 22, 61, 174, or 26...
example 3
Cellular Immune Responses to Human HSJ1, HDJ1, or HDJ2 Peptides Homologous or not Homologous to E. coli hsp dnaJ
[0108]In order to evaluate whether human homologous peptides could be the target of cross-recognition leading to self reactivity, human peptides derived from regions of HSJ1, HDJ1, or HDJ2 having sequence homology with E. Coli dnaJ were studied. SFMC of 14 patients with oJIA presenting a SI to the recombinant E. coli hsp dnaJ greater than 3 were restimulated for 96 hr with autologous cells in presence or absence of human peptide 2, 3, or 5, which are homologs of bacterial peptide 4; human peptide 20, 21, or 23, which are homologs of bacterial peptide 22; or human peptide 164, 167, or 176, which are homologs of bacterial peptide 174. SFMC proliferative responses, pro-inflammatory cytokine production, and anti-inflammatory IL-4 levels were overall similar between the peptides derived from human dnaJ proteins and the homologous bacterial peptides.
[0109]Table 3 shows the proli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| TC | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| hydrophobic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com