Fluorescent Protein Sensors of Post-Translational Modifications
a technology of fluorescent protein and post-translational modification, which is applied in the direction of fluorescence/phosphorescence, instruments, peptide sources, etc., can solve the problems of poor kinetics, destabilized fluorescence under quenching conditions, and destabilized fluorescence under quenching conditions, and achieve the effect of increasing or decreasing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
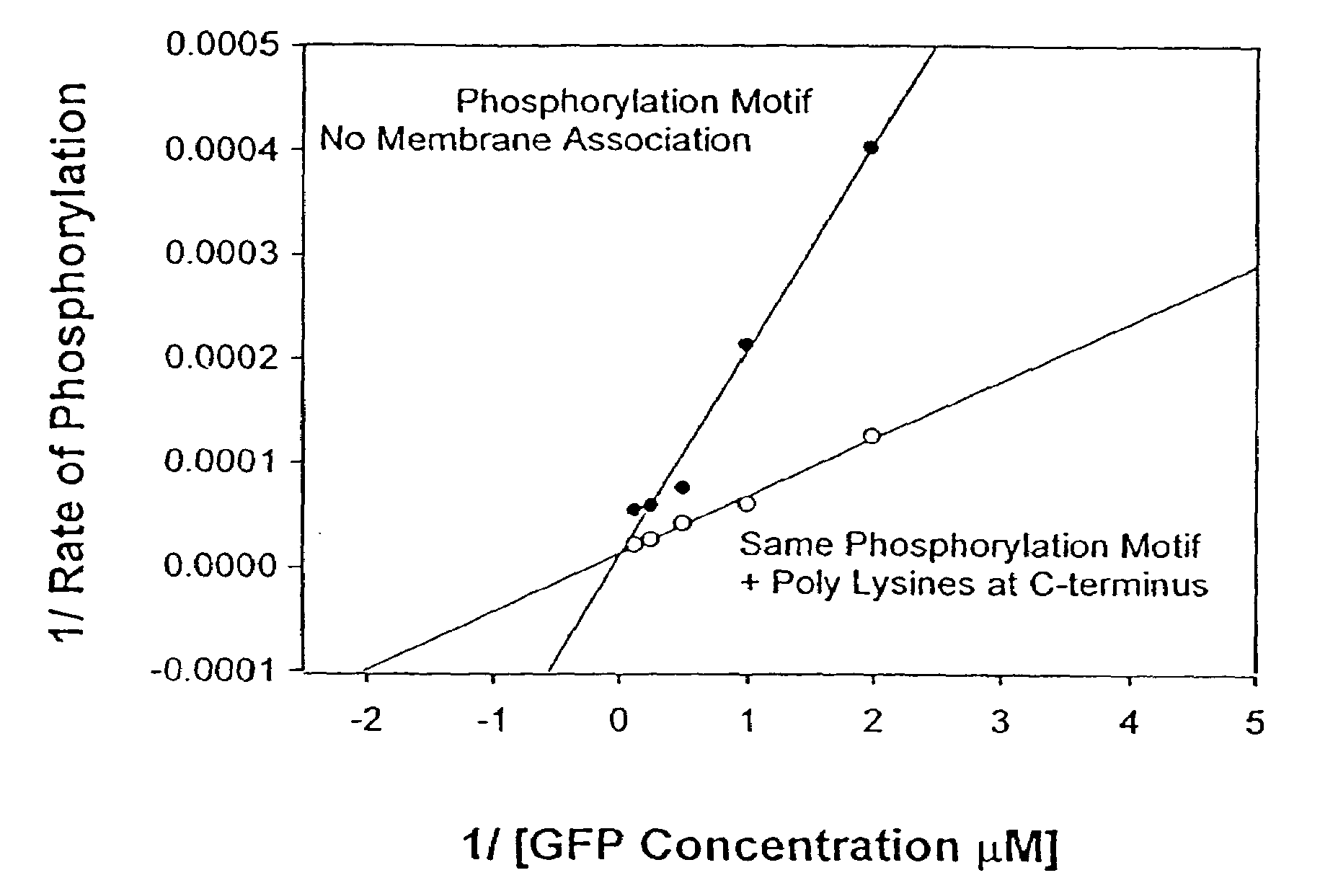
A. Phosphorylation Sites Located in the Amino Acid Sequence of Aequorea GFP Remote in the Primary Amino Acid Sequence Form the N-Terminus
[0134]Potential sites for phosphorylation were chosen at or close to positions in GFP that had previously been identified on the basis of mutagenesis experiments to exert significant effects on fluorescence, or which had a higher probability of surface exposure based on computer algorithms. For example, in mutant H9, Ser 202 and Thr 203 are mutated to Phe and Ile, respectively, creating a large change in spectral properties. Therefore, in one mutant, 199 RRLSI (SEQ ID NO:18), a potential site of phosphorylation was created around Ser 202, whose phosphorylation would significantly affect the fluorescent properties of the parent molecule. Similarly, the amino acids located at positions 72 and 175 have been implicated in increased folding efficiency of GFP at higher temperatures and were made into potential sites of phosphorylation in separate mutants...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com