Platelet promoting protein and the usage thereof
a platelet and protein technology, applied in the field of biomedicine, can solve the problems of short shelf life of platelets, life-threatening low level of blood platelets, allergic reactions among recipients, etc., and achieve the effects of increasing the amount of platelets in the circulating blood, monitoring bleeding times, and stimulating significantly the formation of platelets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0028] Isolation of PPP that Interacts with the Extracellular Domain of MPL by Using a Yeast Two-Hybrid System
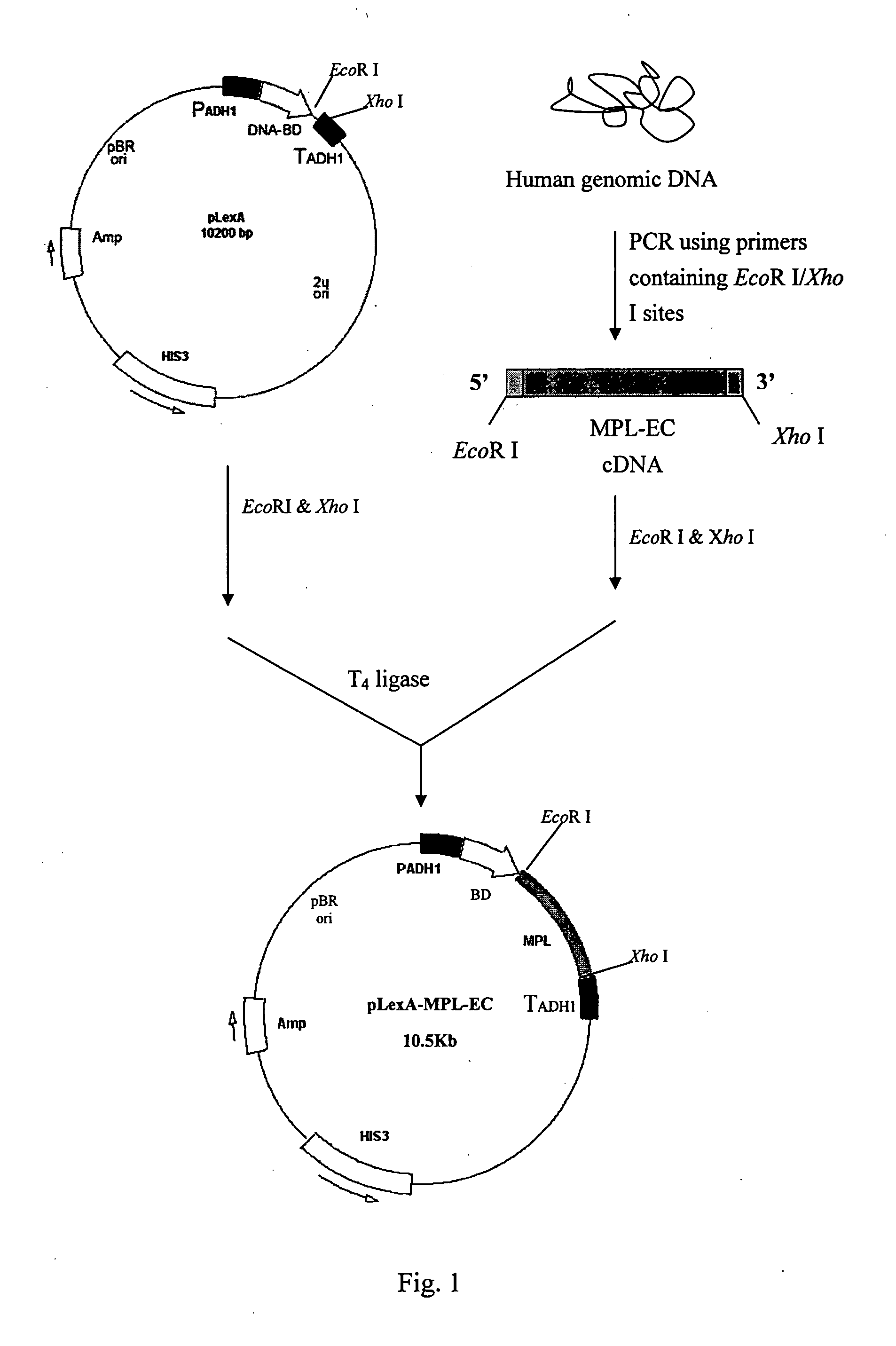
[0029] 1.1 Construction of a Bait Protein Plasmid pLexA-MPL-EC
[0030] The primers MPLEC-F and MPLEC-R, with EcoRI and XhoI incorporated, were synthesized based on the sequence of MPL-EC as follows:
MPLEC-F:5′-CCGGAATTCCAAGATGTCTCCTTGCTGGCATCAGA-3′;MPLEC-R:5′-CCGCTCGAGTTATCCGACCACGAGCTCCAGGG-3′∘
[0031] MPL-EC was PCR amplified with using the total human DNA as the template as indicated in FIG. 1. The PCR reaction mixture of a total 50 μl contained 1×PCR reaction buffer, 5 μM MPLEC-F, 0.5 μM MPLEC-R, 1 μg human total DNA, 2U Taq DNA polymerase (Fermentas), 50 μM dATP, 50 μM dTTP, 50 μM dCTP, 50 μM dGTP, 1.5 mM MgCl2. The PCR program used was: 94° C., 5 min; then 30 cycles of 94° C., 1.5 min, 55° C., 1 min, 72° C., 2 min; with an additional of 72° C., 10 min at the end of the program. The resultant PCR product of approximately 1,450 bps long was separated by and purified from ...
example 2
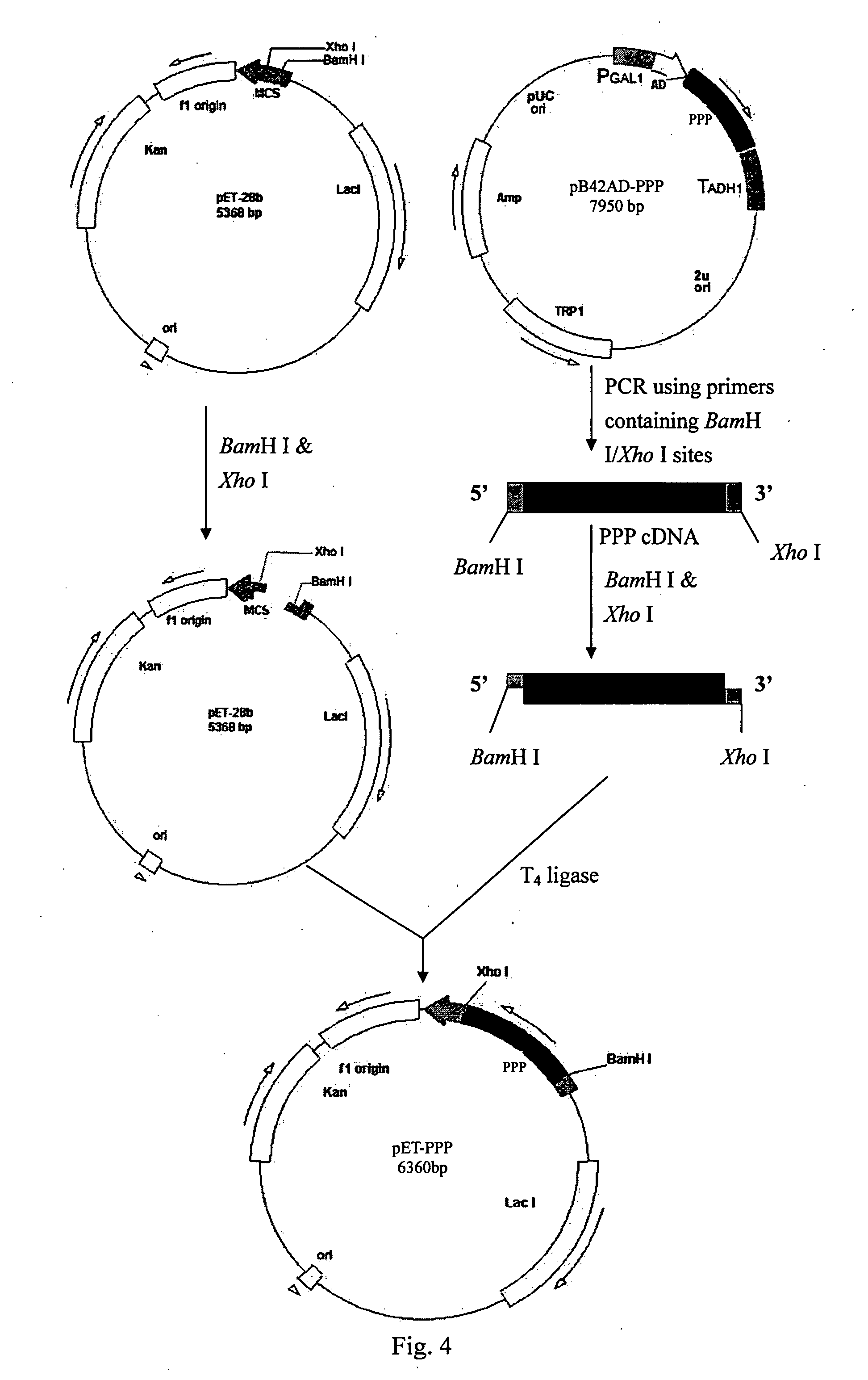
Construction of PPP Expression Vector pET-28b and the Expression and Purification of PPP
[0035] PPP was cloned into His-tag containing expression vector pET-28B (Novagen) (FIG. 3). The expressed protein His-PPP carried six continuous histidine residues at the N-terminus and can be purified by affinity chromatography.
[0036] pET-28b contains multiple cloning sites. The primes PPP-F and PPP-R were designed based on the restriction sites on the vector and the cDNA sequence of PPP:
PPP-F:5′-CGGGATCCGATGGGCGGAGAGCAGGAGGAGGA-3′,containing BamHI (underlined);PPP-R:5′-CCGCTCGAGCTAGTTGAATTTAGCCTTGGAAA-3′,containing XhoI (underlined).
[0037] PPP DNA was amplified with using pB42AD-PPP DNA as the template. The PCR reaction mixture of a total 50 μl contained 1×PCR reaction buffer, 0.5 μM PPP-F, 0.5 μM PPP-R, 1 μg pB42AD-PPP DNA, 2U Taq DNA polymerase (Fermentas), 50 μM dATP, 50 μM dTTP, 50 μM dCTP, 50 μM dGTP, 1.5 mM MgCl2. The PCR program used was: 94° C., 5 min; then 30 cycles of 94° C., 1 mi...
example 3
His-PPP Stimulates Platelet Formation in BALB / c Mice
[0039] The procedures of protein injection and venous platelet measurement were based on Kaushansky et al., Nature, Vol. 369, 1994, 565-568 with modifications.
[0040] The His-PPP purified as described at Example 2 was diluted into a stock solution of 10 μg / ml with PBS containing 0.1% BSA and used for the injection. Thirty normal male BALB / c mice of 6-7 week old were divided randomly into three groups of ten mice each. The first group was subcutaneously injected with 10 μg / kg His-PPP once per day for seven consecutive days; the second group was subcutaneously injected with 50 μg / kg His-PPP once per day for seven consecutive day; and the third group (control) was subcutaneously injected with PBS containing 100 μg / ml of BSA once per day for seven consecutive days. Twenty μl venous blood was collected from a small lateral cut in the tail vein on day 0, 4, 7, 10, 13, 16 and 19. The platelets were counted using of an F-820 Sysmex electr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com