Methods and compositions of novel triazine compounds
a triazine compound and compound technology, applied in the field of triazine compounds, can solve the problems of chronic inflammation leading to complications and ongoing system damage, endothelial damage, vascular complications,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
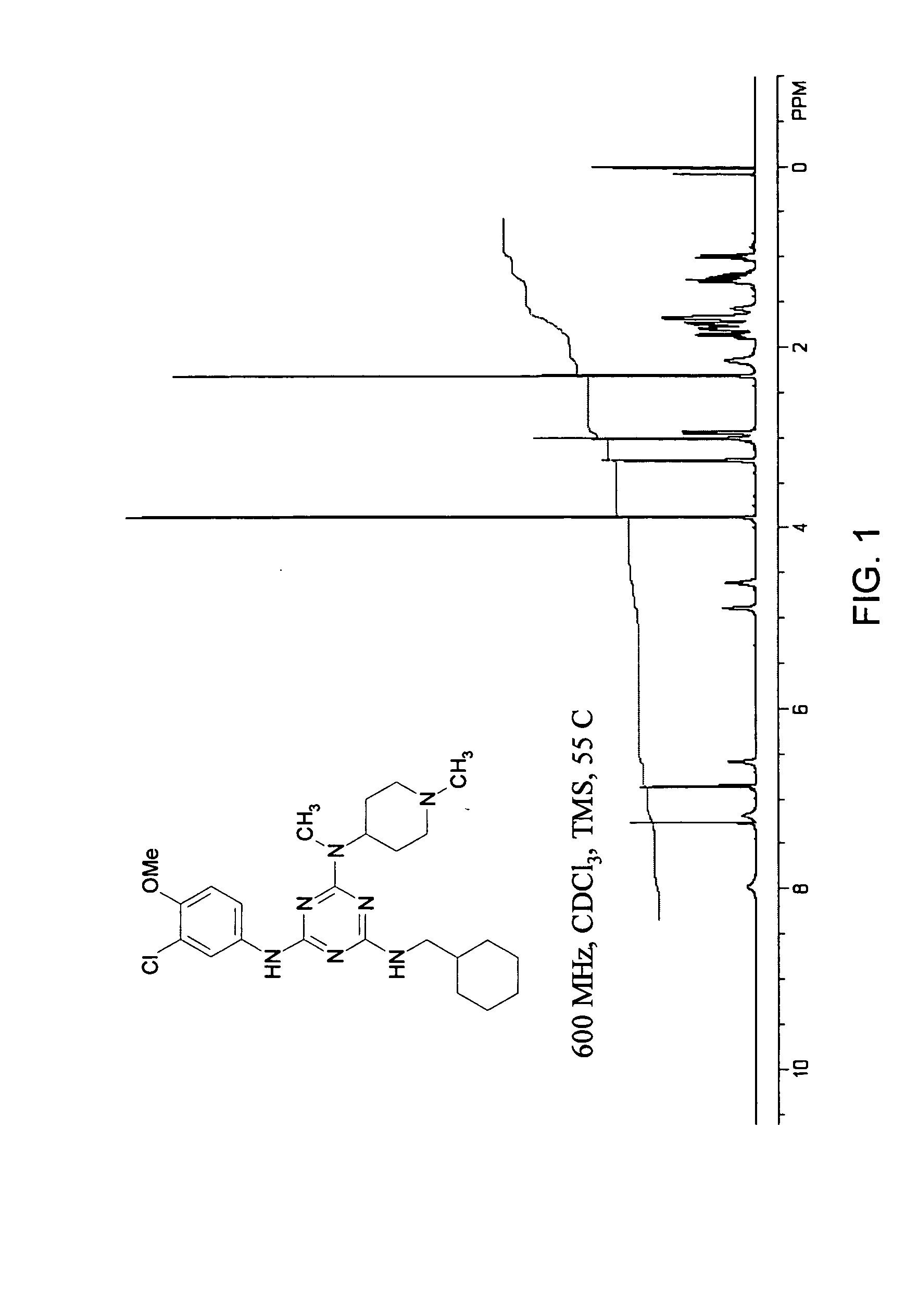
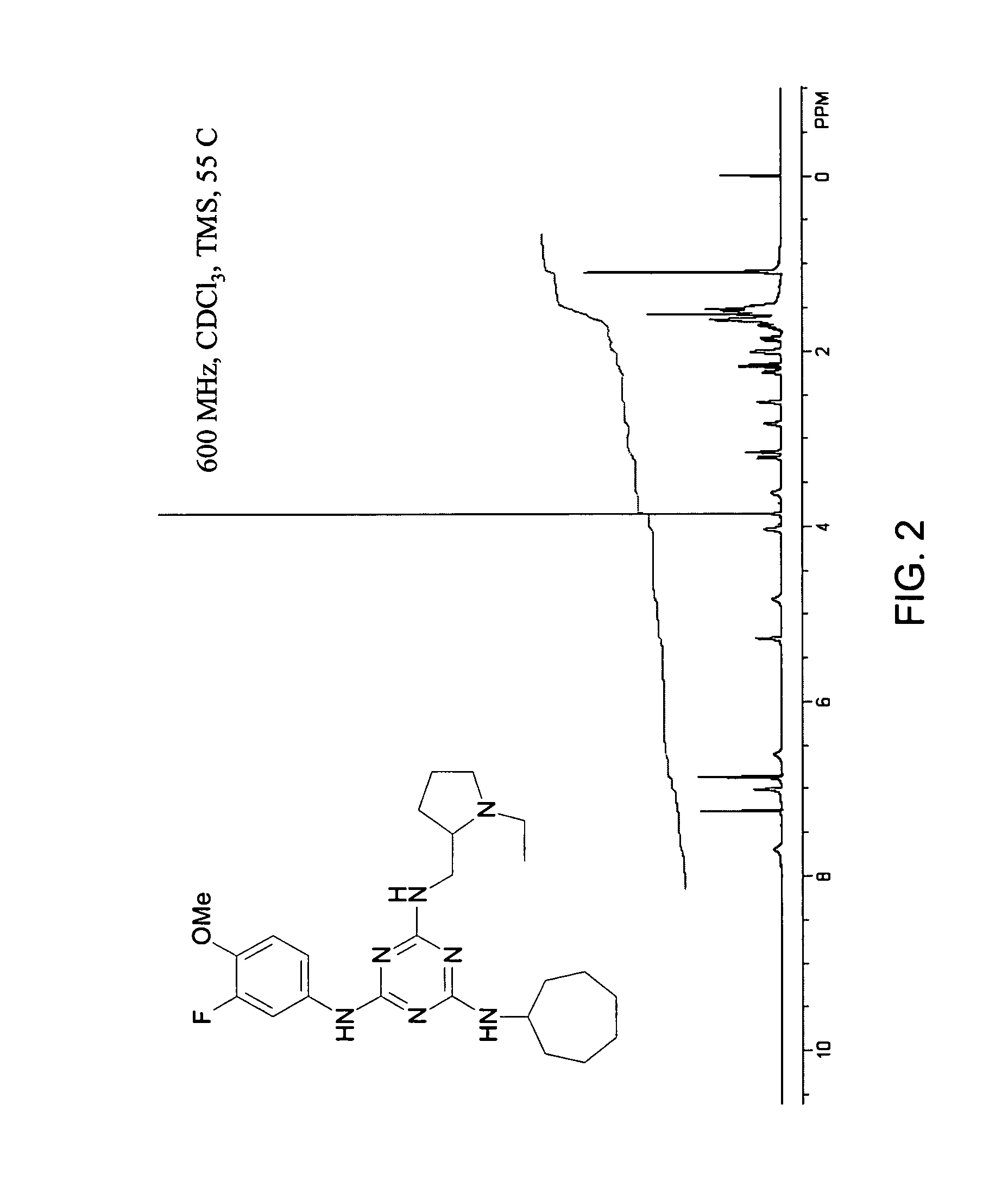
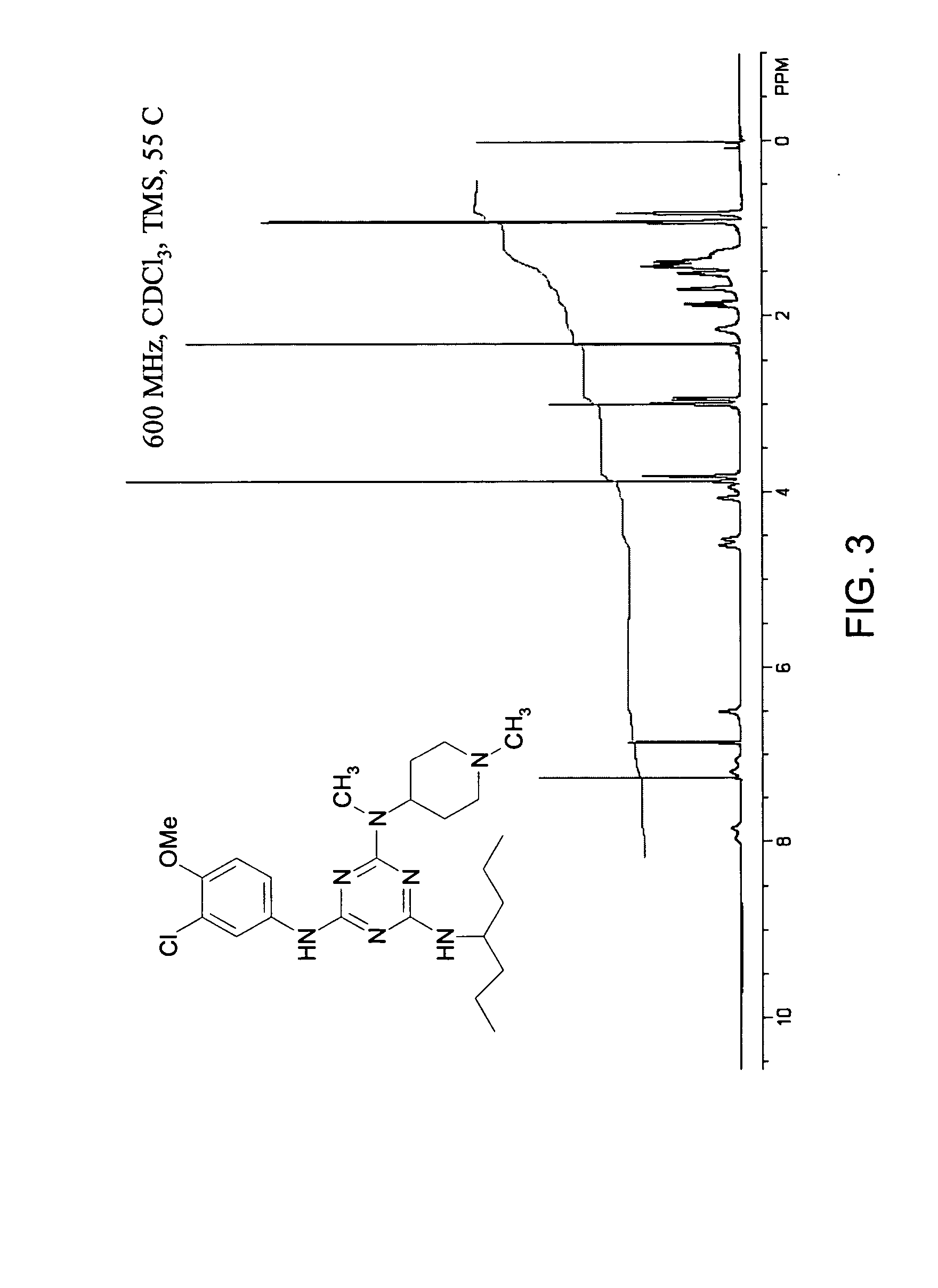
General Synthetic, Purification, Characterization, and Spectroscopic Procedures
[0457] General Synthetic Procedures. Room temperature is defined as an ambient temperature range, typically 20-25° C. An ice bath (crushed ice / water) temperature is defined as a range, typically −5 to 0° C. Temperature at reflux is defined as ±15° C. of the boiling point of the primary reaction solvent. Overnight is defined as a time range of 8-16 hours. Vacuum filtration (water aspirator) is defined as range of 5-15 mm Hg. Dried under vacuum is defined as using a high vacuum pump as a range of 0.1-5 mm Hg. Neutralization is defined as a typical acid-based neutralization method and measured to a pH 6-8 range using pH-indicating paper. Brine is defined as a saturated aqueous sodium chloride. Nitrogen atmosphere is defined as positive static pressure of nitrogen gas passed through a Drierite column with an oil bubbler system. Concentrated ammonium hydroxide is defined as an approximately 15 M solution.
[04...
example 2
General Methods for Parallel Synthesis
[0470] Examples 3-5 describe the synthetic procedures for the preparation of the “library” of N2, N4, N6-tris(amino)-1,3,5-triazines which was prepared based on the strategy of changing only one pendant amino group per synthesis, and based on the parent structure 95 shown below, where each compound in the library contains two of the pendant groups in 95.
[0471] The library was divided into three subgroups, and all three subgroups are presented in Table 2. Library I (compounds 1-50) includes compounds having unchanged cycloheptylamino and [(1-ethyl-2-pyrrolidinyl)methyl]amino substituents, with various groups being permuted at the remaining triazine amino position, prepared by Method A as presented in Example 3. Library II (compounds 51-75) includes compounds having unchanged [(1-ethyl-2-pyrrolidinyl)methyl]amino and (3-fluoro-4-methoxyphenyl)amino substituents, with various groups being permuted at the remaining triazine amino position, prepar...
example 3
Parallel Synthetic Method A, for Library I Compounds
[0472] The following reaction scheme presents the general reagents and conditions for parallel synthetic method A used for the compounds of Table 2 which designate Method A.
Reagents and conditions: (a) ArNHR, DIEA, CH3CN / 1,4-dioxane, −11 C, 1 h (b) cycloheptylamine, DIEA, CH3CN / 1,4-dioxane, rt, overnight (c) 2-(aminomethyl)-1-ethylpyrrolidine, DIEA, CH3CN / 1,4-dioxane, 80 C, 15
[0473] A stock solution of cyanuric chloride (0.542 M) in 1,4-dioxane was prepared and 1 mL of this solution (containing 100 mg or 0.542 mmol) was dispensed into each of 50 barcoded 40 mL vials. These solutions were cooled to about −11° C. (freezing) using a J-KEM block connected to a circulating cooler. Meanwhile, individual solutions of each aryl amine ArNHR (specified as Monomer 1 in Table 2, 0.542 mmol) and disopropylethylamine (DIEA) (77 mg / 104 μL, 0.596 mmol) in 1 mL of CH3CN were prepared. (For HCl salts, 204 μL DIEA (approx. 2.1 equiv) was used.) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total thickness | aaaaa | aaaaa |
| total thickness | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com