Metod of modulation of interaction between receptor and ligand
a technology of receptor and ligand, which is applied in the direction of antibacterial agents, antibody medical ingredients, peptide/protein ingredients, etc., can solve the problems that the relation to known biological functions of ncam is not well understood, and achieve the effect of stimulating neurite outgrowth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Production of Recombinant Proteins
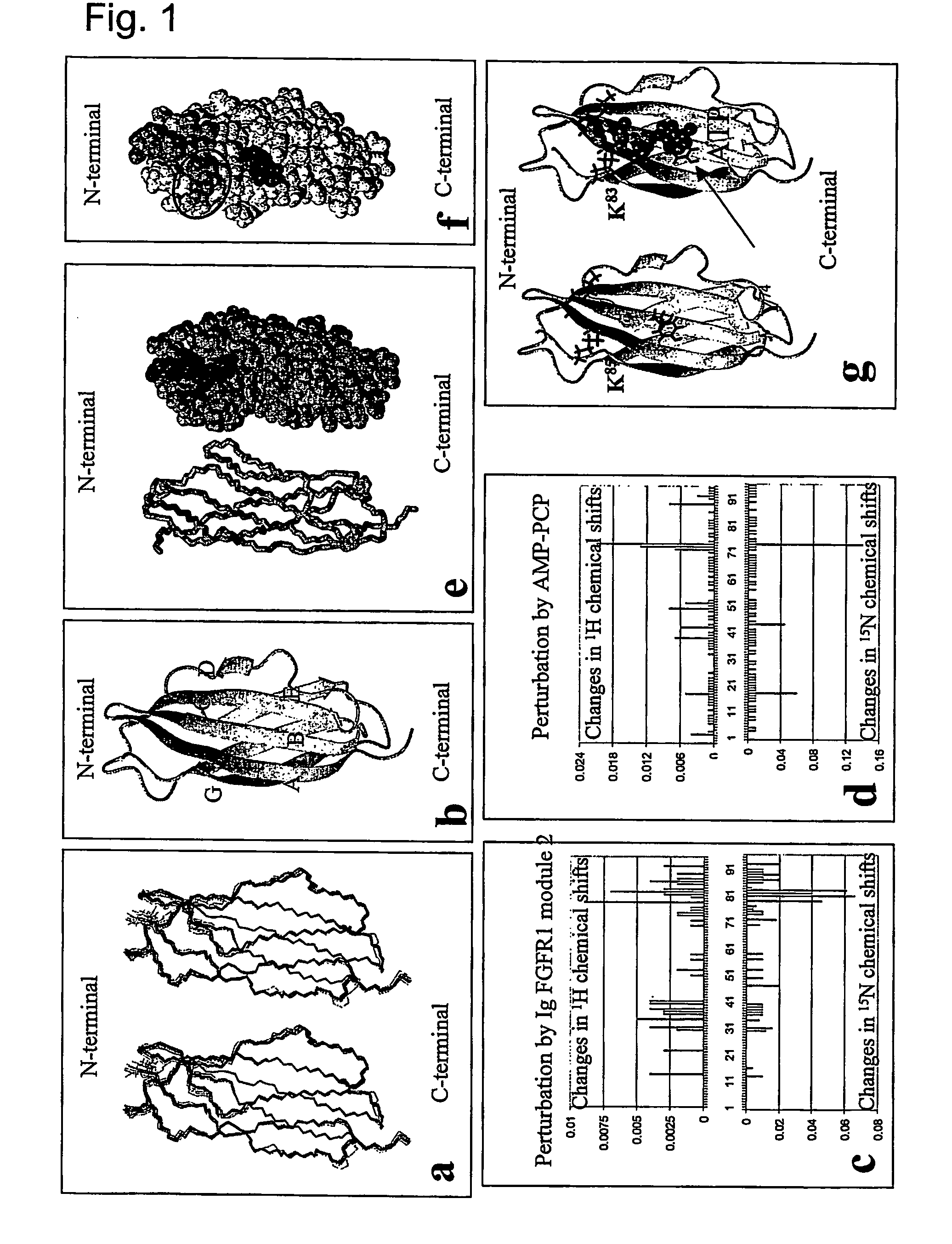
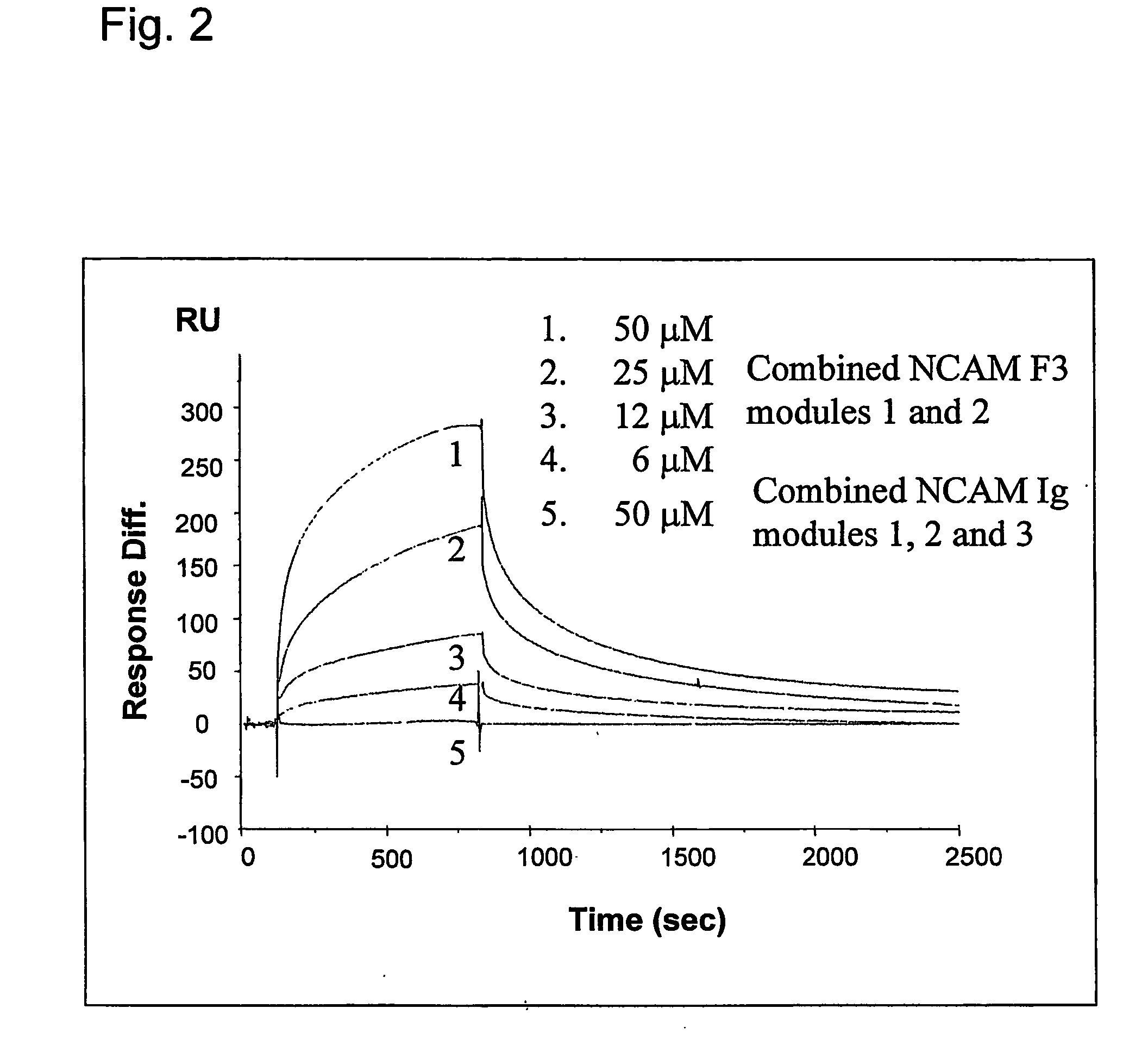
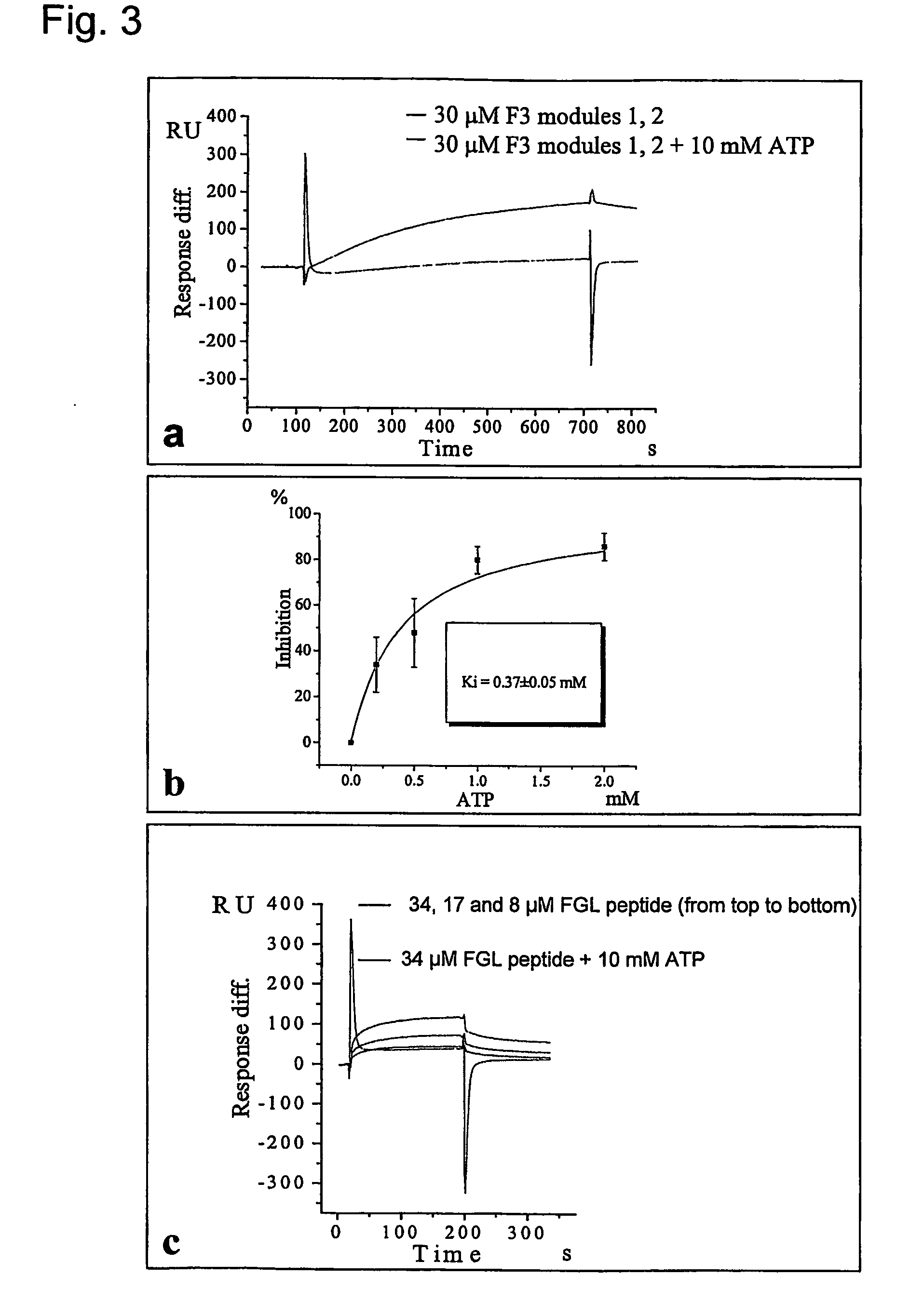
[0352] The NCAM F3 modules 1, 2 (without expression of exons a, MG), and FGFR1 Ig modules 2, 3 were produced using rat NCAM cDNA and mouse FGFR1 (IIIC isoform) cDNA. The F3 module 1 and the combined F3 modules 1, 2 consist of AGHHHHHH and amino acids 507-611 and 507-705 of NCAM (swissprot p13596), respectively. The F3 module 2 consists of AG and amino acids 612-705 of NCAM (swissprot p13596), and is sequentially numbered from 1 to 96, A being numbered 1. The FGFR Ig module 2 consists of AGHHHHHH and amino acids 140-251 of FGFR (swissprot p16092). The FGFR Ig module 3 and the combined Ig modules 2, 3 consist of RSHHHHHH and amino acids 249-365 and 141-365 of FGFR (swissprot p16092), respectively. The F3 modules and the FGFR Ig module 2 were expressed in a KM71 strain of yeast P. pastoris (Invitrogen, USA) as described (Thomsen et al., 1996). The FGFR Ig module 3 and modules 2, 3 were expressed in Drosophila S2 cells (Invitrogen, USA) according to t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell death | aaaaa | aaaaa |
| Adhesion strength | aaaaa | aaaaa |
| Plasticity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com