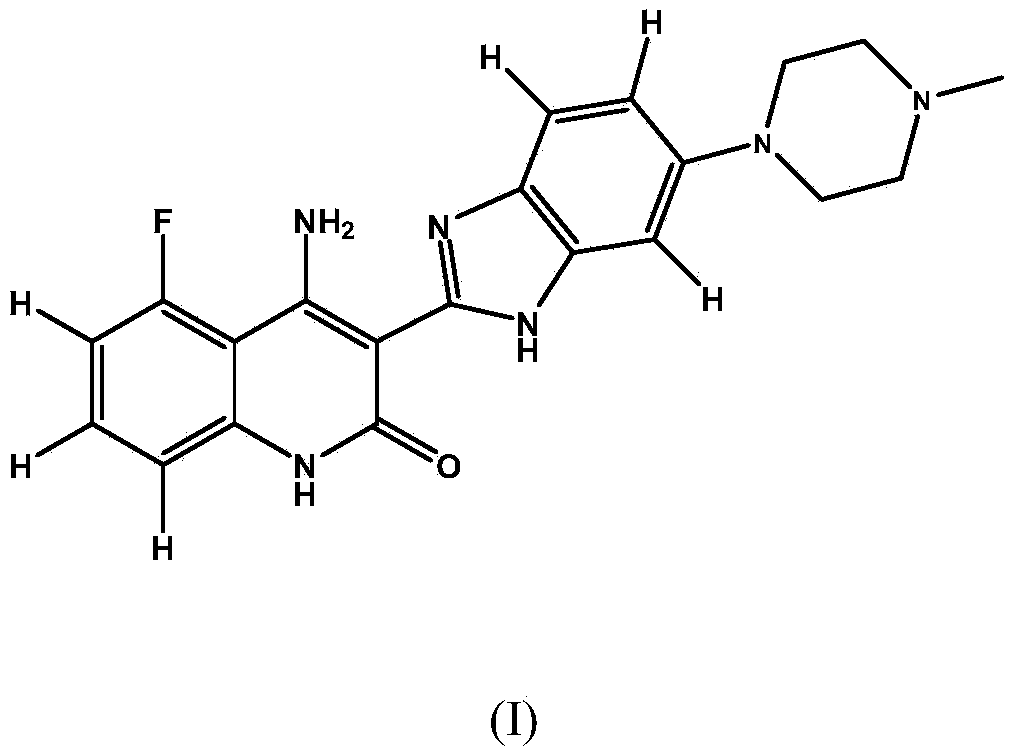
FGFR and ligands thereof as biomarkers for breast cancer in HR positive subjects
A biomarker, FGFR1 technology, applied in biochemical equipment and methods, microbial measurement/testing, instruments, etc., can solve the problems of tumor proliferation and poor prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A clinical study testing the efficacy of dovitinib in FGFR1-amplified and non-amplified metastatic breast cancer
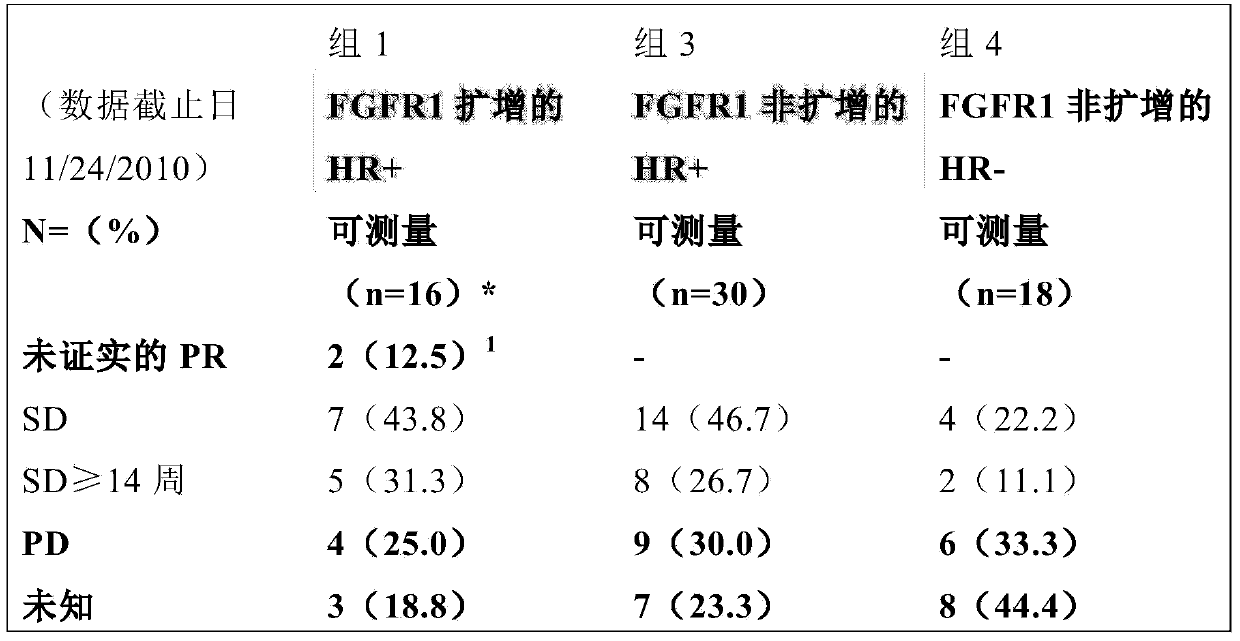
[0038] A multicenter, open-label, phase 2 trial of dovitinib was completed to evaluate the clinical activity of dovitinib and test its clinical efficacy in FGFR1-amplified and non-amplified metastatic breast cancer. The efficacy and safety of dovitinib were studied in 4 groups of patients with metastatic breast cancer: (Group 1: FGFR1+, HR+), (Group 2: FGFR1+, HR-), (Group 3: FGFR1-, HR+), (Group 4: FGFR1-, HR-). Patient selection was done based on FISH / CISH for FGFR1 (truncated ≥6 gene copies). Dovitinib (500 mg) was administered once daily based on a 5-day on / 2-day off regimen. The primary endpoint was RECIST best overall response rate (in pts) with detectable disease at each external radiology review.
[0039] standard constrain
[0040] 1. Patients with histologically confirmed breast cancer and a clinical diagnosis of IBC based on the appearance of...
Embodiment 2
[0064] Example 2: Clinical Study Testing the Efficacy of an FGFR1 Inhibitor (Dovitinib) in FGFR1 Amplified Breast Cancer Patients
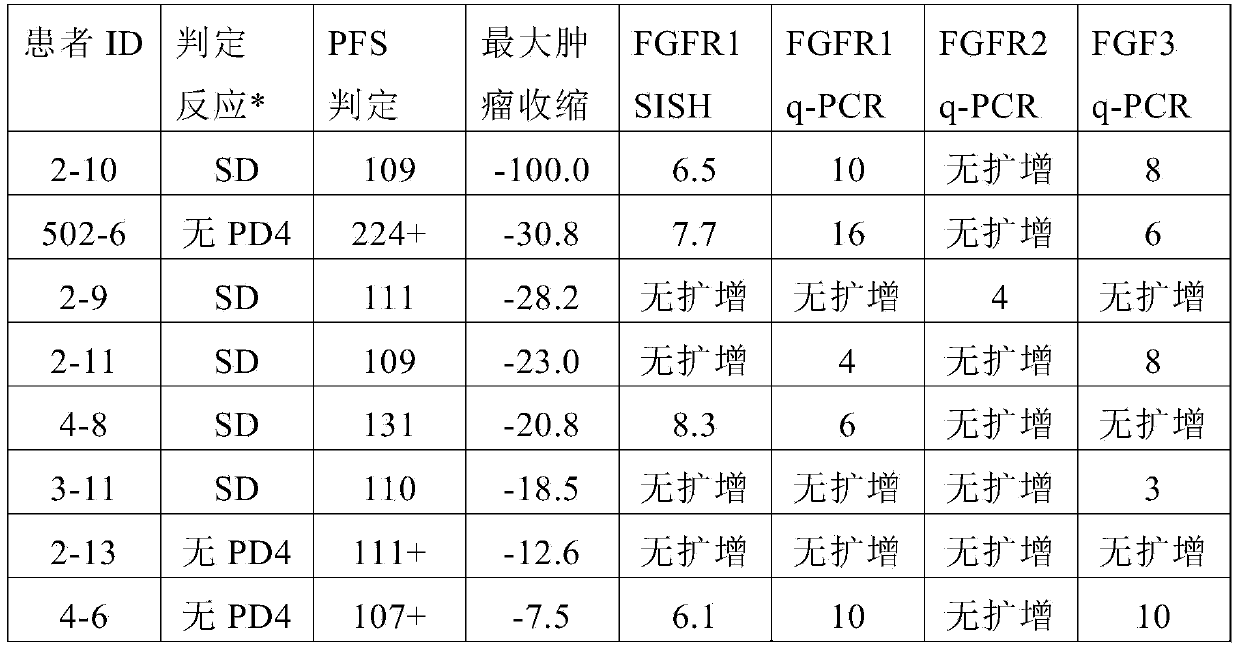
[0065] After analyzing the dovitinib clinical study results from patient groups 1, 3, and 4, an exploratory analysis was also performed to further evaluate the clinical response in patients with tumors harboring other gene amplifications. Amplification of FGF ligands (FGF3, FGF4, FGF19) and FGFR2 gene amplification were also completed according to the previously defined protocol. The protocol describes a copy number analysis method for the FGFR1 and FGF3 genes using ABI's predesigned TaqMan TM Copy number determination.
[0066] Method summary
[0067] for FGFR1 and FGF3 Copy number assays were purchased from Applied Biosystems and compared with Copy number reference assays were run together in a duplex real-time polymerase chain reaction (PCR). A copy number assay detects the target gene of interest (in this case FGFR1 or FGF3), and a refe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com