Suspension formulation of interferon
a technology of suspension and interferon, which is applied in the direction of peptide/protein ingredients, implants, prostheses, etc., can solve the problems of difficult design of protein formulations that are stable at elevated temperature for a long time, and the patient is not easily tampered with by the patient, etc., and achieves the effects of reducing the risk of infection, and improving the safety of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
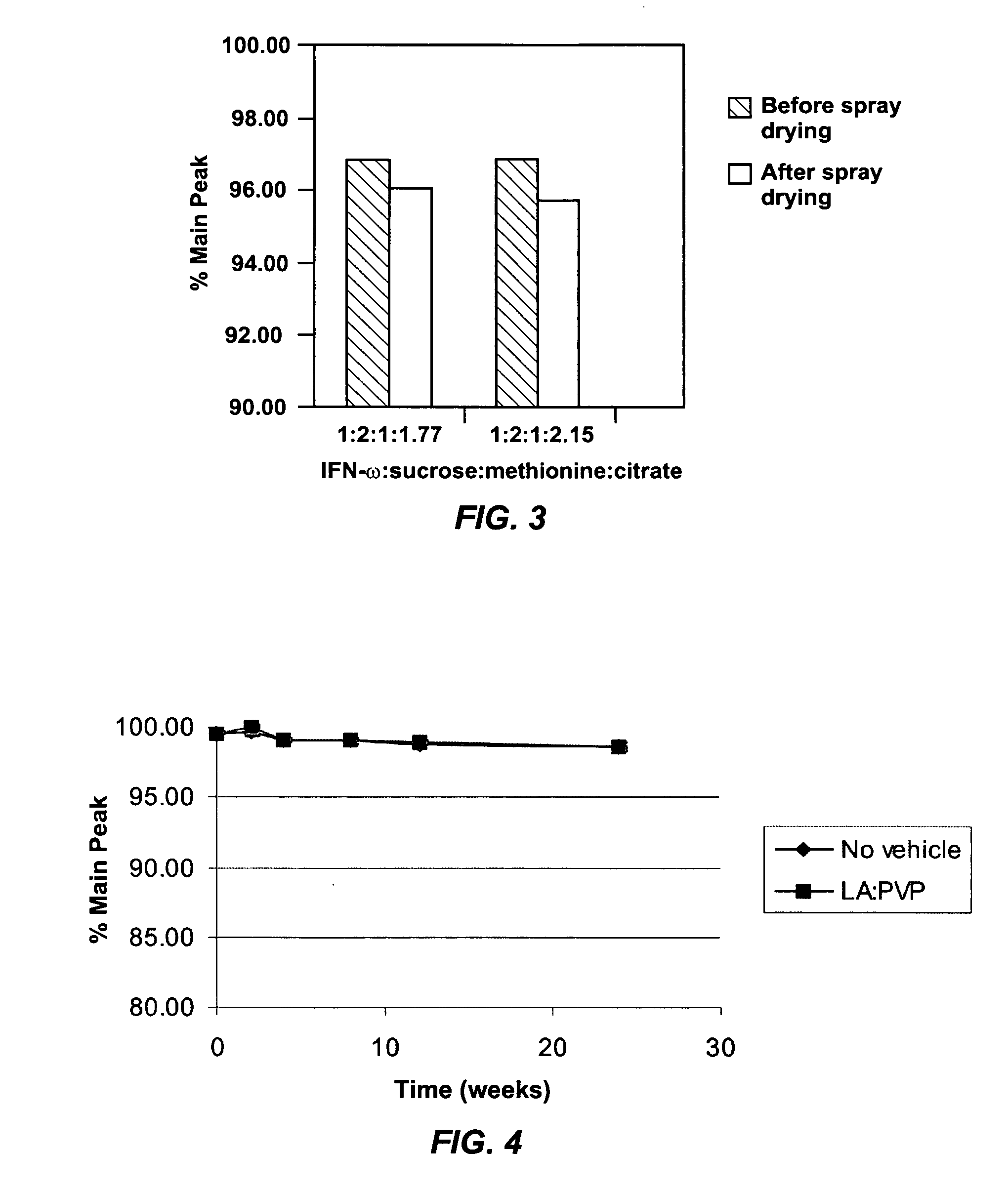
[0045] A bulk solution of IFN-ω was obtained as a frozen solution having a concentration of approximately 5 mg / ml. The IFN-ω solution was dialyzed against 25 mM citrate solution (pH 6.0). Sucrose and methionine in citrate solution were added to the dialyzed IFN-ω to make final IFN-ω:sucrose:methionine:citrate in a ratio of 1:2:1:1.77. The solution was spray dried as described above. The average particle size was 4-5 μm. The spray solution and spray dried particles were analyzed using RP-HPLC. The first two bars of FIG. 3 show percent main peak for the spray solution and spray dried particles of this example. Percent main peak refers to the fraction of IFN-ω detected that is in a monomeric form and does not appear to be chemically degraded in any form
example 2
[0046] A bulk solution of IFN-ω was obtained as a frozen solution having a concentration of approximately 5 mg / ml. The IFN-ω solution was dialyzed against 25 mM citrate solution (pH 6.0). Sucrose and methionine in citrate solution were added to the dialyzed IFN-ω to make final IFN-ω:sucrose:methionine:citrate in a ratio of 1:2:1:2.15. The solution was spray dried as described above. The average particle size was 4-5 μm. The spray solution and spray dried particles were analyzed using RP-HPLC. The second two bars of FIG. 3 show percent main peak for the spray solution and spray dried particles of this example.
example 3
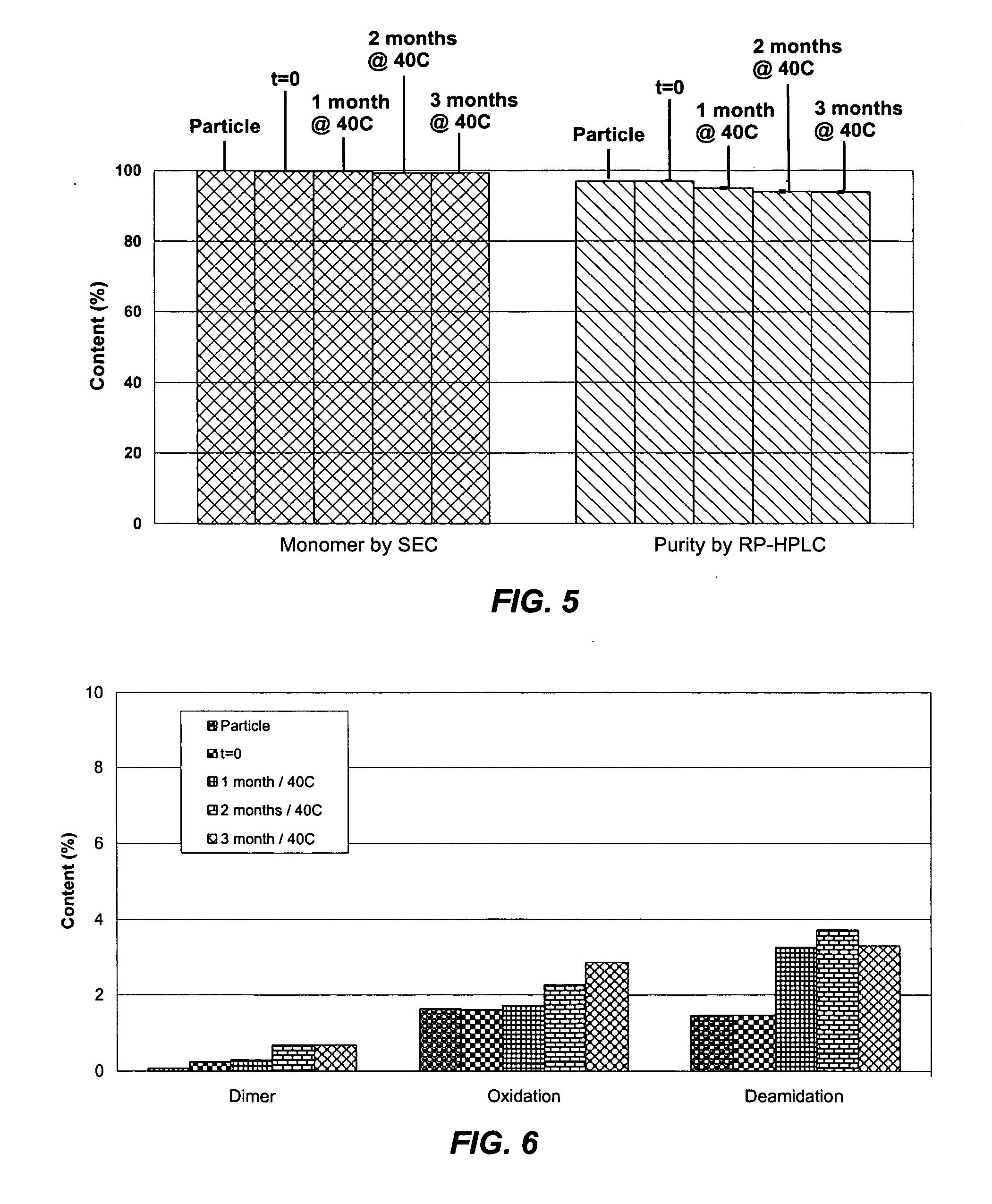
[0047] A bulk solution of IFN-ω was obtained as a frozen solution having a concentration of approximately 5 mg / ml. The IFN-ω solution was dialyzed against 25 mM citrate solution (pH 6.0). Sucrose and methionine in citrate solution were added to the dialyzed IFN-ω to make final IFN-ω:sucrose:methionine:citrate in a ratio of 1:2:1:2.2 at IFN-ω concentration of 3.3 mg / mL. The solution was spray dried as described above. The spray dried particles were evaluated using RP-HPLC and SEC at various timepoints during storage. The results are shown in Tables 3 and 4 below.
TABLE 3SECRP-HPLCProteinMonomerMain PeakContentTemperatureTime(Standard(Standard(Standard(° C.)(months)Deviation)Deviation)Deviation)A (n = 15)0100.00 (0.01) 96.26 (0.39)16.11 (0.21)B (n = 3)40199.85 (0.00)96.99 (0.19)16.47 (0.07)C (n = 3)40299.90 (0.01)95.85 (0.01)16.16 (0.22)D (n = 3)40399.93 (0.02)96.45 (0.35)16.51 (0.22)E (n = 3)40699.88 (0.00)95.24 (0.12)17.01 (0.13)F (n = 3)25699.93 (0.01)96.20 (0.10)17.14 (0.14)G (n ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| storage temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com