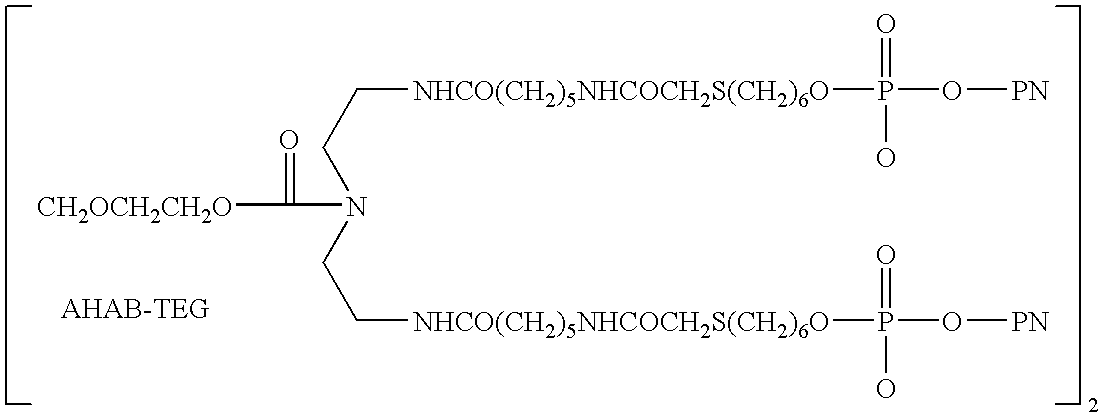Methods of treating lupus based on antibody affinity and screening methods and compositions for use thereof
a technology of antibody affinity and lupus, applied in the field of antibody-mediated pathologies, can solve the problems of relatively poor overall survival and treatment regimens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Inhibition of Binding of Anti-dsDNA Antibodies to DNA by LJP 394
[0172] After determining the presence of anti-ds DNA antibodies in patients using a Farr assay, a competitive Farr assay was used to measure the affinity of anti-dsDNA antibodies found in sera from patients with SLE to LJP 394. In addition, the assay was used to measure the affinity of anti-dsDNA antibodies found in sera from three animals models of SLE (BXSB mice, NZB×NZW F1 mice, and MRL / lpr mice).
[0173] The Farr assay used 125I-labeled recombinant dsDNA (Diagnostic Products Corporation, Los Angeles, Calif.) that was combined with the anti-dsDNA antibodies found in sera from patients with SLE or from the mouse models of SLE. Anti-dsDNA antibodies were obtained from serum samples of donors with SLE collected through a volunteer donor program. Blood samples were drawn, serum harvested, aliquots made, labeled, and stored frozen at −70° C. until used. In this assay, 25 μl of patient's serum was added to 75 μl of Tris bu...
example 2
Identifying SLE Patients by Affinity Assay
[0179] SLE patients were readily identified by measuring the binding affinity of sera from SLE patients to LJP 394 dsDNA epitope using the surface plasmon resonance assay described below. IgG fractions from 10 SLE patients serum samples and 10 normal patient serum samples were evaluated for binding to LJP 394 ds DNA epitope with a non-competitive direct affinity assay (BIACORE™; Piscataway, N.J.), as disclosed herein. FIG. 2 illustrates that the SLE samples all showed saturable binding to the epitope while normal samples showed low binding to the LJP 394 epitope and low specific binding.
example 3
Determination of Titer-Weighted Average Affinity of Antibodies for Conjugate and Response to Treatment with Conjugate
[0180] An assay using surface plasmon resonance was developed to directly measure a titer-weighted average affinity of antibodies from SLE patients for the conjugate LJP 394. Surface plasmon resonance is used to quantify the fractional saturation of antigen with antibody. This assay was adapted so that it measured the average affinity of the IgG population of LJP 394.
[0181] Materials and Methods
[0182] Reagents. Streptavidin CM5 chips, HBS buffer (0.01 M HEPES pH 7.4, 0.15 M NaCl, 3 mM EDTA and 0.005% (v / v) surfactant P20) were obtained from BIACORE AB (Piscataway, N.J.).
[0183] LJP 394 is composed of four 20-mer dsDNA epitopes that are covalently attached to a triethyleneglycol-based platform by a thiol linkage. The DNA epitope was composed of 5′-(CA)10-3′ strands annealed to complementary GT strands, with biotin attached at the free 5′ ends of the GT strand. Bioti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com