Inhibition of histone deacetylase as a treatment for cardiac hypertrophy
a technology of histone deacetylase and cardiac hypertrophy, which is applied in the field of development biology and molecular biology, can solve the problems of cardiac hypertrophy, which is still not fully understood, and achieve the effects of improving one or more symptoms of cardiac failure, increasing exercise capacity, and increasing blood ejection volum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
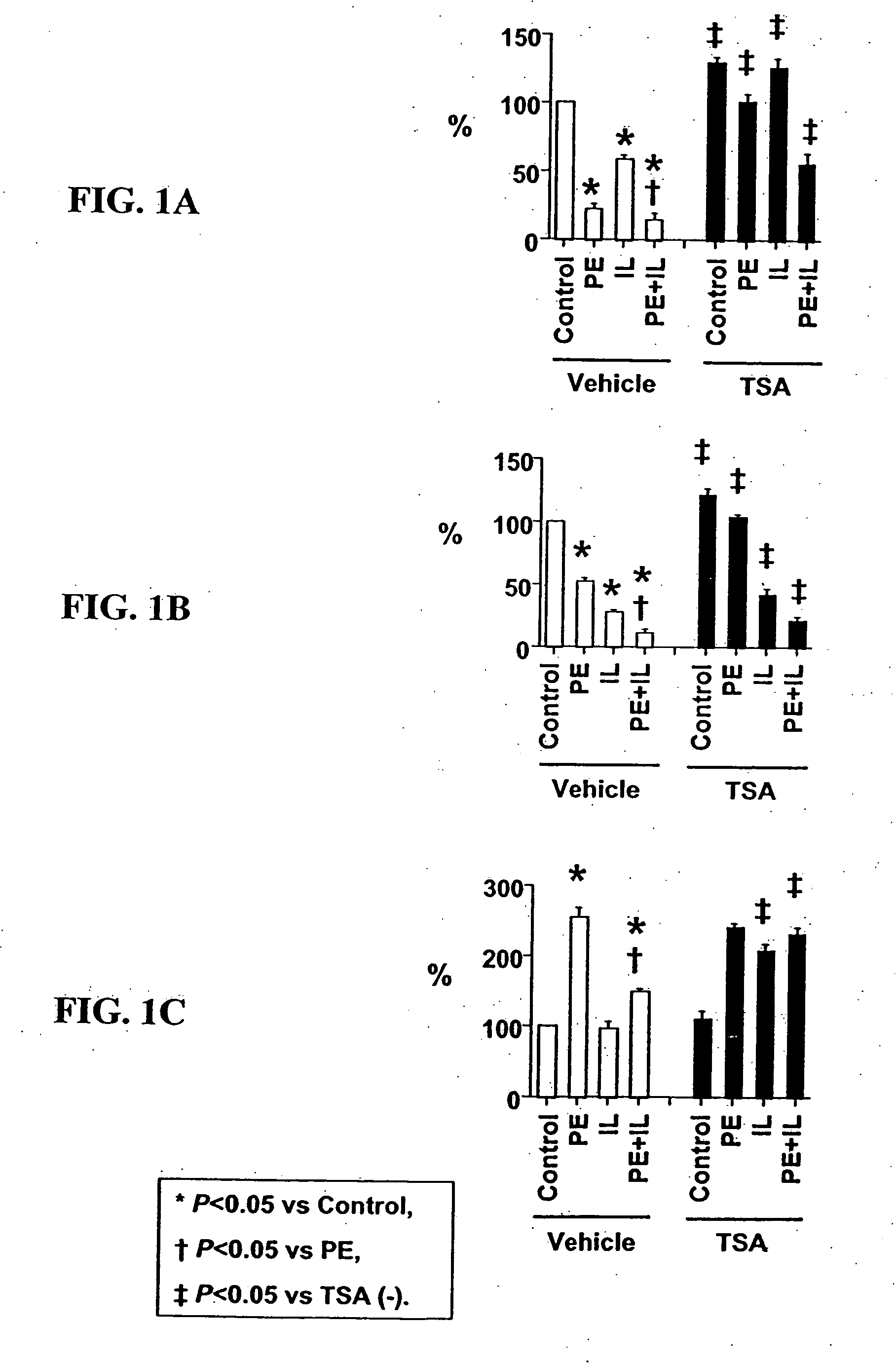
[0099] Cell culture. Ventricular myocytes from one-day-old rats were plated at low density in MEM with 5% calf serum, and studied in serum-free MEM. Cultures were treated with PE (#P6126, Sigma-Aldrich Corp., St. Louis, Mo.), IL-1 (#501-RL, R&D Systems, Minneapolis, Minn.), TSA (#GR-309, BIOMOL Research Laboratories, Inc., Plymouth Meeting, Pa.) or their vehicles (ascorbic acid for PE; bovine serum albumin for IL-1; dimethyl sulfoxide for TSA).
[0100] Detection of acetylated lysine residues. Total cell extract was subjected to Western blot analysis using antibodies from Cell Signaling Technology (Beverly, MA) (acetylated lysine antibody: #9441, acetylated histone H3 antibody at Lysine 9: #9671, acetylated histone H3 antibody at Lysine 23: #9674, histone H3 antibody: #9712). Nuclear localization of acetylated histone H3 was examined by immunostaining using the same antibodies.
[0101] Quantification of myocyte hypertrophy and myocyte-specific mRNA expression. Gr...
example 2
Materials and Methods
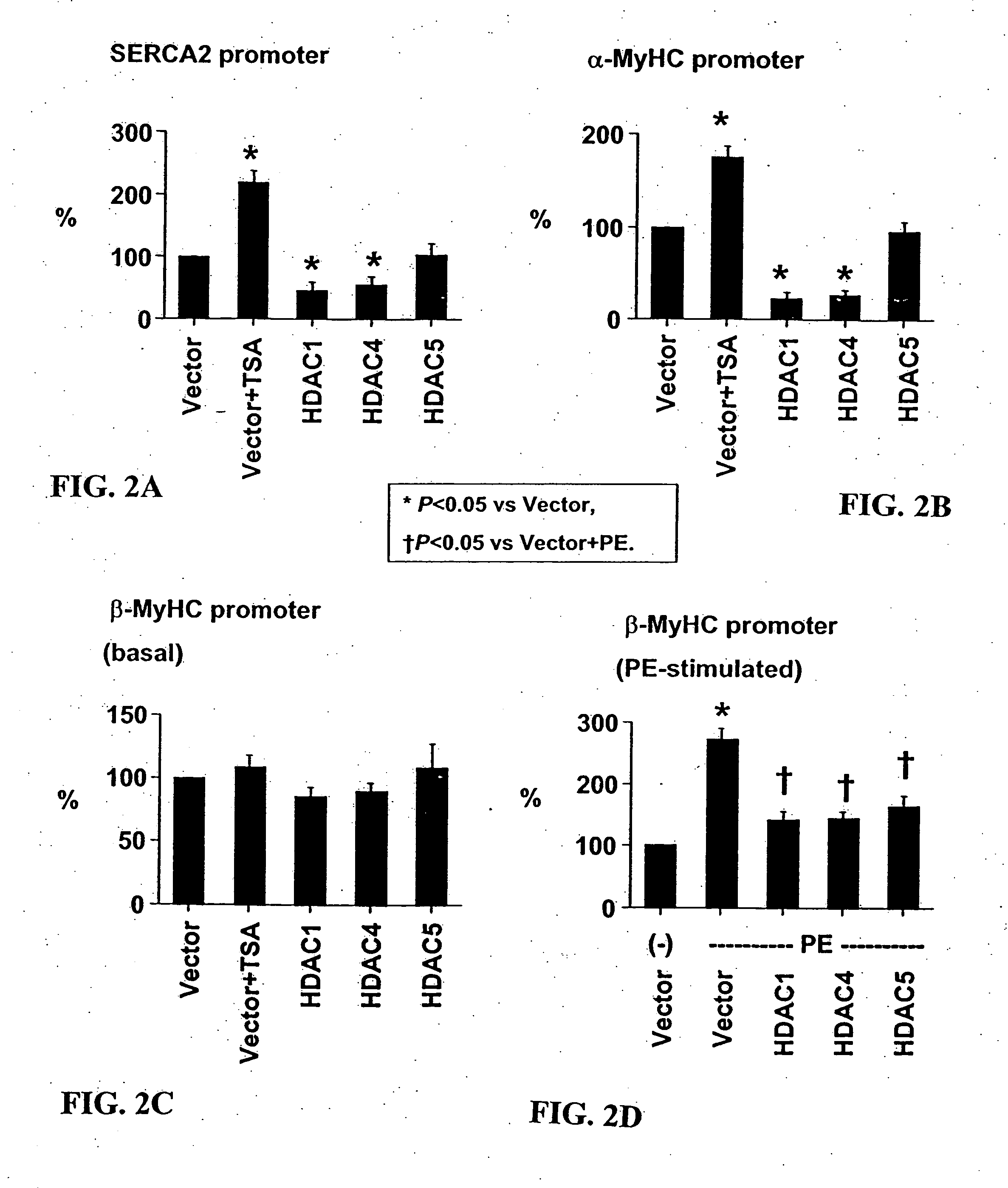
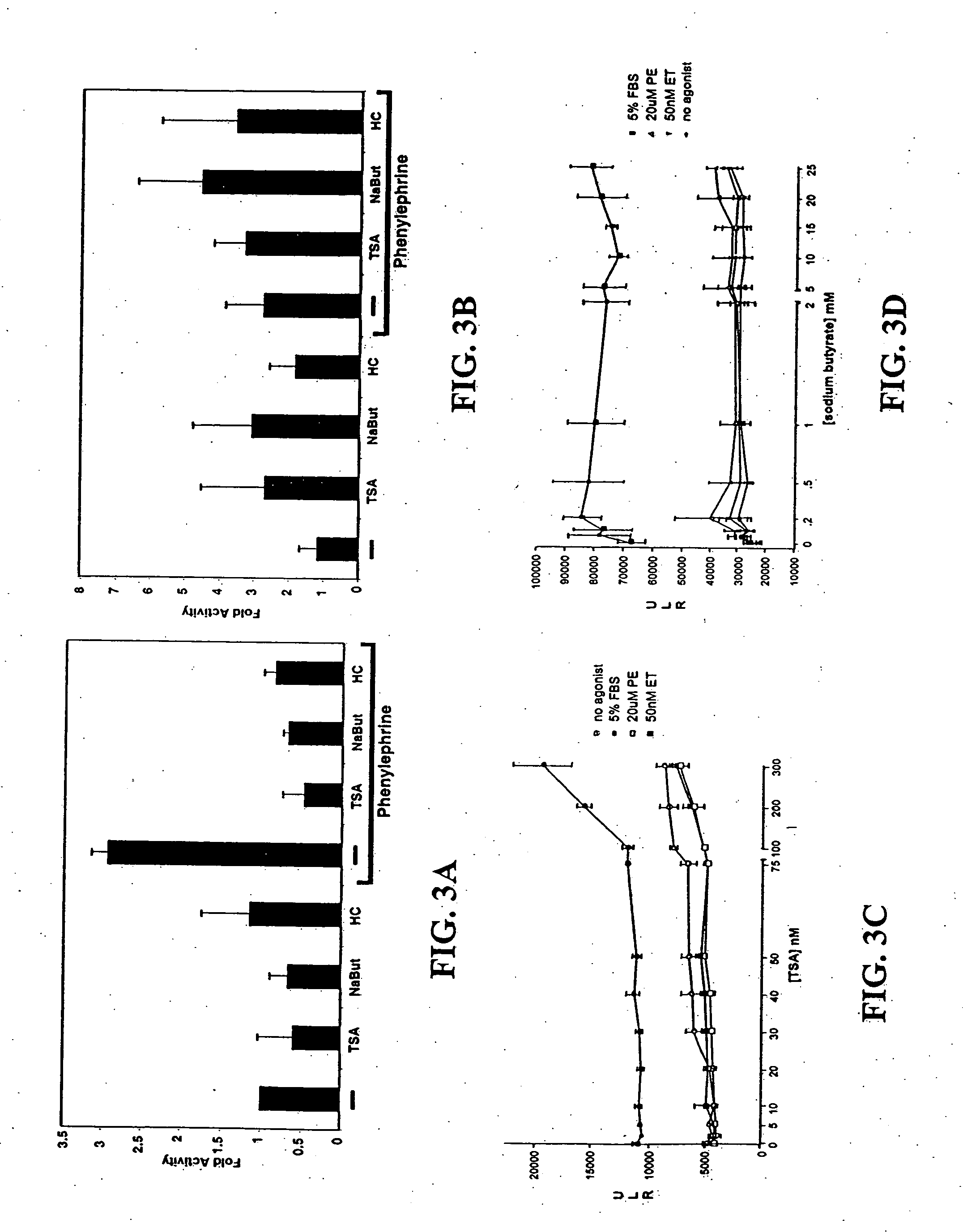
[0106] Cardiomyocyte isolation. Hearts were harvested from 15-day timed-pregnant female Sprague-Dawley rats (Harlan, Houston, Tex.). After mincing in phosphate-buffered saline, cardiomyocytes were isolated from successive digestion fractions of 0.1% (w / v) Pancreatin (Sigma, St. Louis, Mo.) solution. Fractions were collected; resuspended in plating medium, pooled; and then plated for 2 hours to separate fibroblasts from cardiomyocyte population. Suspended cells were recollected and plated in 6-well dishes at 1×106 cells / well for transfection and immunofluorescence experiments, and 2×106 cells in 10 cm dishes for RNA analysis.
[0107] Transcription assay. Twenty-four hours after plating, cells were transfected with total of 1 μg / ml for 5 hrs using Lipofectamine Plus reagent (Invitrogen, Carlsbad, Calif.). Transfected cells were incubated 24 hrs then treated for an additional 24 hrs. Cells were lysed and their lysates assayed with the Luciferase Assay System (Prom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| cell size | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com