Glycosyltransferases for biosynthesis of oligosaccharides, and genes encoding them
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
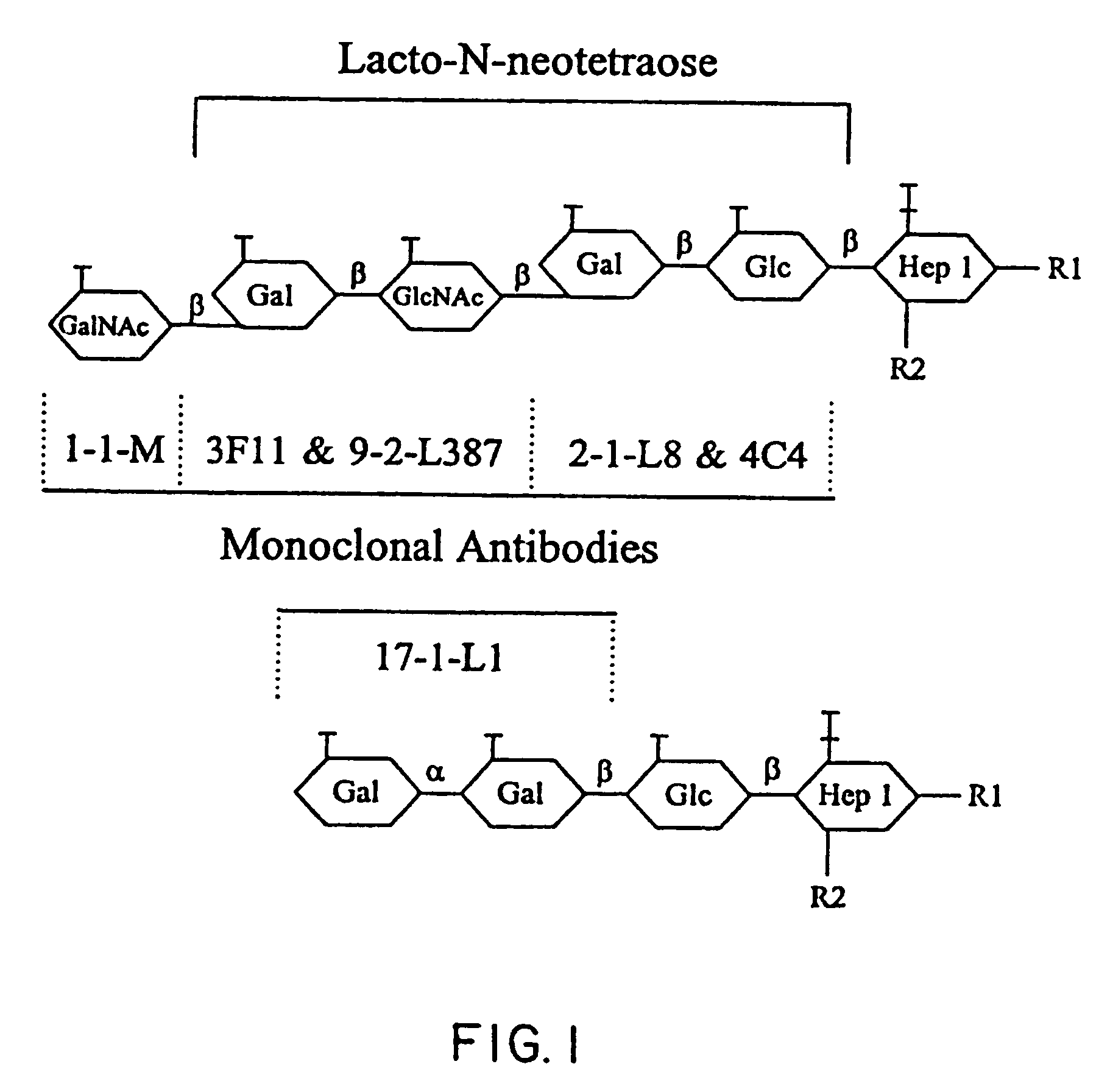
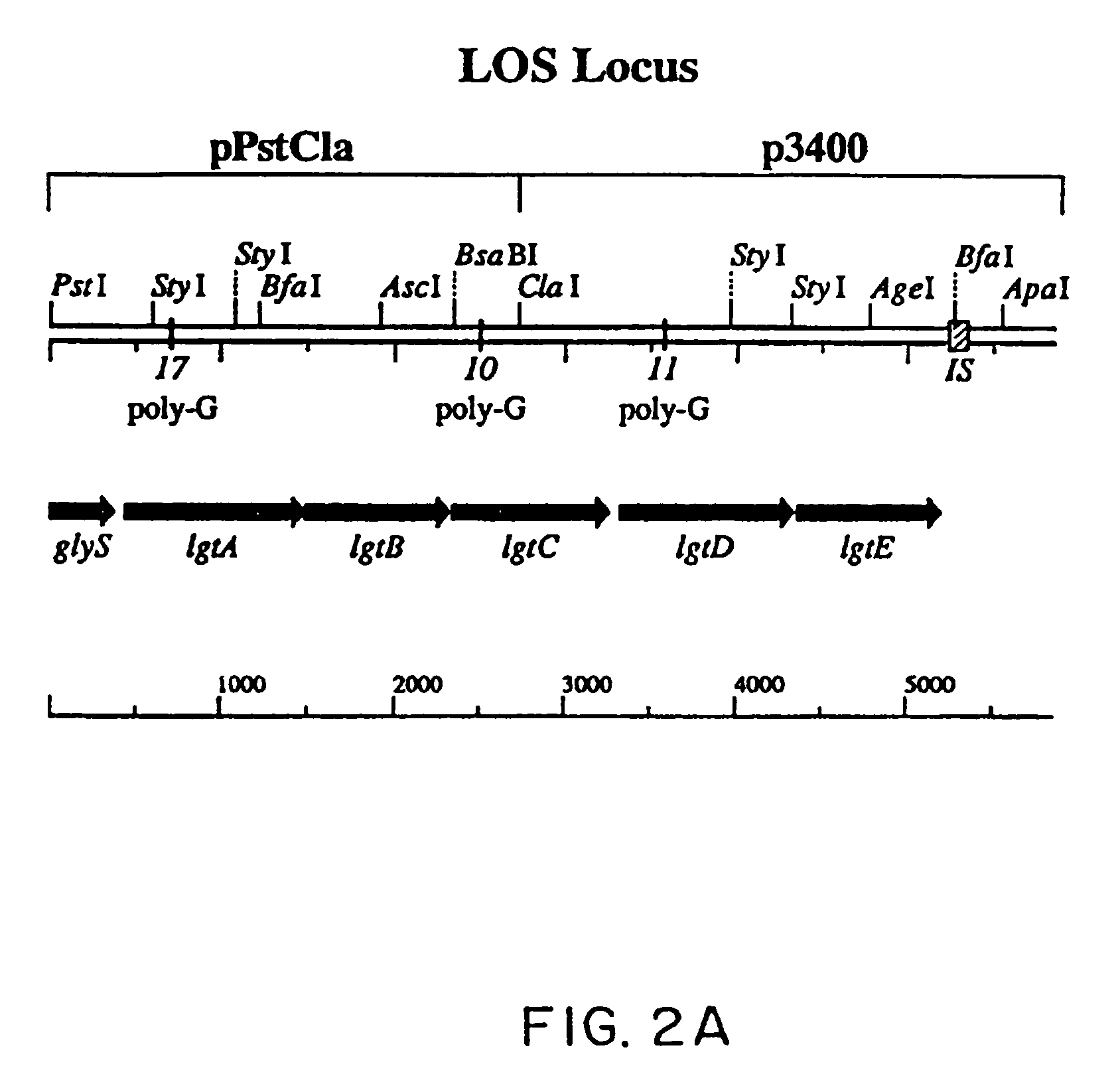
[0115] This Example describes a locus in Neisseria gonorrhoeae strain F62 containing five genes. Four of the genes are responsible for the sequential addition of the GalNAcβ1→3Galβ1→4GlcNAcβ1→3Galβ1→4 to the substrate Glcβ1→4Hep→R of the inner core region (Yamasaki et al., 1991, Biochemistry 30:10566). The fifth gene is involved with the addition of the α-linked galactose residue in the biosynthesis of the alternative LOS structure Galα1→4Galβ1→4Glcβ1→4Hep→R (John et al., 1991, J. Biol. Chem. 266:19303). The DNA sequence analysis revealed that the first, third and fourth reading frames contained poly-G tracts which in strain F62 were respectively 17, 10 and 11 bp. Thus, three of the LOS biosynthetic enzymes are potentially susceptible to premature termination by reading-frame changes, as has been reported for the gonococcal pilC genes (Jonsson et al., 1991, EMBO J. 10:477; Rudel et al., 1992, Molec. Microbiol. 6:3439). It is likely that these structural features are responsible for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com