Vascular therapeutics
a technology of vascular therapy and vascular artery, applied in the field of vascular therapy, can solve the problems affecting the ability of anti-sense molecules, and not necessarily translating, so as to achieve the effect of reducing the level of c-jun mrna
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
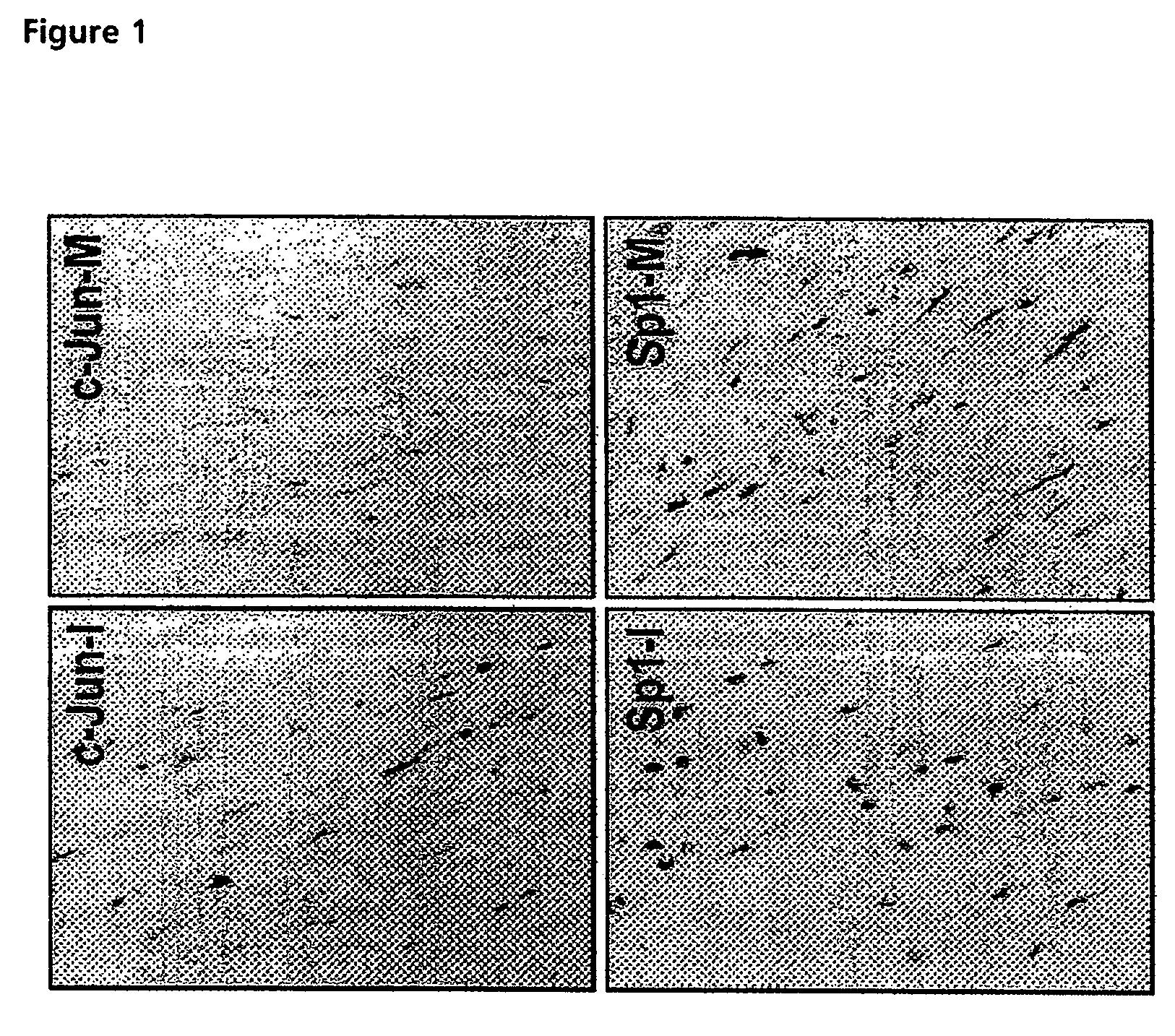
demonstrated c-Jun expression by smooth muscle cells in the human atheromatous lesion (FIG. 1). c-Jun is poorly, if at all, expressed by smooth muscle cells in the normal media. In contrast, the zinc finger transcription factor Sp1 is expressed in both the intima and media (FIG. 1).
[0050] Neointima formation is a characteristic feature of common vascular pathologies, such as atherosclerosis and post-angioplasty restenosis, and involves smooth muscle cell proliferation.
[0051] In addition to its expression by smooth muscle cells, the present inventors have also demonstrated that c-Jun is linked to the complex process of angiogenesis and is involved in ocular angiogenesis as is reviewed in Adamis et al., (1999)29. Ocular angiogenesis is responsible for the majority of irreversible blindness in the developed world. This debilitating complication affects all age groups and characterizes such diverse and widespread diseases as trachoma, retinopathy of prematurity, diabetic retinopathy, n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com