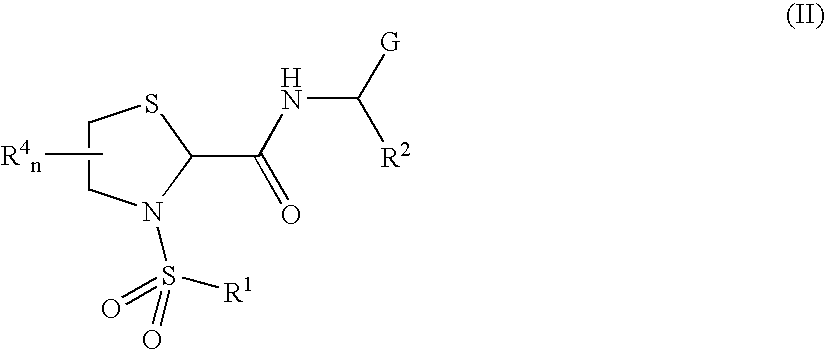
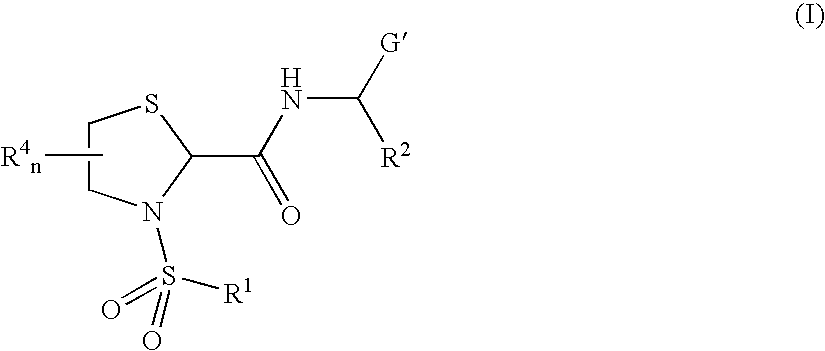
Thiazolidine carboxamide derivatives as modulators of the prostaglandin f receptor
a technology of prostaglandin f and carboxamide, which is applied in the direction of heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problems of inconvenient agents, preterm labor, respiratory depression and cardiac arrest,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
General Protocols for the Solution-Phase Synthesis of 1,3-thiazolidine-2-carboxamide derivatives of general formula (I) e.g., 3-([1,1′-biphenyl]-4-ylsulfonyl)-N-[(1S)-3-hydroxy-1-phenylpropyl]-1,3-thiazolidine-2-carboxamide, (25)-3-([1,1′-biphenyl]-4-ylsulfonyl)-N-[(1S)-3-hydroxy-1-phenylpropyl]-1,3-thiazolidine-2-carboxamide (2R)-3-([1,1′-biphenyl]-4-ylsulfonyl)-N-[(1S)-3-hydroxy-1-phenylpropyl]-1,3-thiazolidine-2-carboxamide (2S)-3-(1,1′-biphenyl-4-ylsulfonyl)-N-[(R)-phenyl(pyridin-2-yl)methyl]-1,3-thiazolidine-2-carboxamide.
Strategy 1:
[0484] N-methyl morpholine (NMM) (3.240g, 2.5eq, 32.15 mmol) was added to a solution of a compound of general formula (VIII) (Intermediate 8, 4.50 g, 1 eq, 12.86 mmol), e.g., 3-([1,1′-biphenyl]-4-ylsulfonyl)-1,3-thiazolidine-2-carboxylic acid, in dry THF (100 ml) and the reaction mixture was cooled down to −25° C. To the reaction mixture was then added drop wise, over a period of 5 minutes, isobutyl chloroformate (1.84 g, 1.05eq, 13.50 mmol) in s...
example 2
3-([1,1′-biphenyl]-4-ylsulfonyl)-N-[(1R)-2-hydroxy-1-phenylethyl]-1,3-thiazolidine-2-carboxamide
[0490] Following the general strategies and protocols outlined in Example 1, starting from 3-([1,1′-biphenyl]-4-ylsulfonyl)-1,3-thiazolidine-2-carboxylic acid (Intermediate 8) and commercial (2R)-2-amino-2-phenylethanol, the title compound was obtained in 98% purity by RPLC.
[0491]1H NMR (300 MHz, CDCl3); 2.4-2.9 (m, CH2S, 2H), 3.5-3.7 (m, CH2N, 2H), 3.7-3.9 (m, CH2O, 2H), 4.9 (m, CH, 1H), 5.2 (s, CH, 1H), 7.1-7.9 (m, CH(Ar), 14H); M+(ESI+): 469.2; M−(ESI−) 467.1.
example 3
3-([1,1′-biphenyl]-4-ylsulfonyl)-N-[(R)-phenyl(2-pyridinyl)methyl]-1,3-thiazolidine-2-carboxamide
[0492] Following the general strategies and protocols outlined in Example 1, starting from 3-([1,1′-biphenyl]-4-ylsulfonyl)-1,3-thiazolidine-2-carboxylic acid (Intermediate 8) and (R)-phenyl(2-pyridinyl)methanamine (Intermediate 1), the title compound was obtained in 96% purity by HPLC.
[0493]1H NMR (300 MHz, CDCl3); 2.5-3.0 (m, CH2S, 2H), 3.6-4.0 (m, CH2N, 2H), 5.41 (s, CH, 0.5H), 5.42 (s, CH, 0.5H), 6.07 (m, CH, 1H), 5.2 (s, CH, 1H), 7.1-7.8 (m, CH(Ar), 16H), 7.8-7.9 (m, CH, 1H), 8.5-8.6 (m, CH, 1H); M+(ESI+): 516.3; M−(ESI−) 514.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Nuclear radiation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com