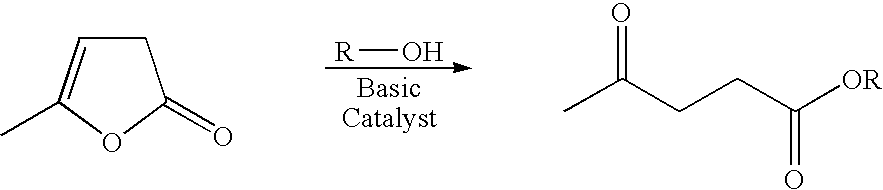
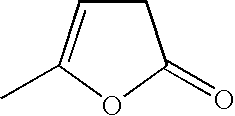
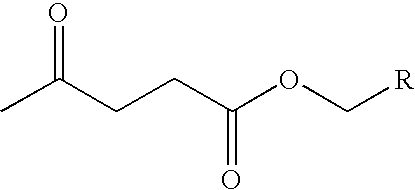
Preparation of levulinic acid esters from alpha-angelica lactone and alcohols
a technology of alpha-angelica lactone and levulinic acid, which is applied in the preparation of ester-hydroxy reactions, organic chemistry, fuels, etc., can solve the problems of limited commercial use of levulinic acid esters and low-cost route to manufacture of these esters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
General Procedure for the Reaction of Alcohols and α-Angelica Lactone
[0057] A 2 cc pressure vessel was charged with 700 mg of a solution consisting of alcohol, α-angelica lactone and 50 mg of a catalyst. The reactor was pressurized with nitrogen and heated to reactor temperature for a specified period of time. The vessel was then cooled, vented and the products analyzed by gas chromatography on a HP-6890 GC (Agilent Technologies; Palo Alto, Calif.) and HP-5972A GC-MS detector equipped with a 25M×0.25MM ID CP-Wax 58 (FFAP) column. The GC yields were obtained by adding methoxyethyl ether as the internal standard.
[0058] The examples described below were performed according to a similar procedure under the conditions indicated for each example.
examples 2-19
[0059]
Reaction of α-Angelica Lactone (AGL) with 1-Butanol (1-BuOH) toProduce Butyl Levulinate (BuLV)N2AGLBuLVExpt.TimeTempPressureConversionSelectivityNo.Basic Catalyst(hrs)(° C.)(MPag)Feedstock(%)(%)2Et3N31005.52AGL (30%) / 1-77.5629.08BuOH (70%)3Et3N11505.52AGL (30%) / 1-89.0345.68BuOH (70%)4Et3N31505.52AGL (30%) / 1-98.8851.31BuOH (70%)5Dabco ™31505.52AGL (30%) / 1-99.2258.01BuOH (70%)620%31505.52AGL (30%) / 1-96.9755.68CsOAc / KA-160BuOH (70%)SiO2720%31505.52AGL (30%) / 1-98.9653.36RbOAc / KA-160BuOH (70%)SiO2820%11505.52AGL (30%) / 1-91.8737.56CsOAc / KA-160BuOH (70%)SiO2920%11505.52AGL (30%) / 1-95.4242.01RbOAc / KA-160BuOH (70%)SiO21020%31005.52AGL (30%) / 1-62.5920.94CsOAc / KA-160BuOH (70%)SiO21120%31005.52AGL (30%) / 1-63.2121.98RbOAc / KA-160BuOH (70%)SiO212Li2CO331005.52AGL (30%) / 1-22.5279.06BuOH (70%)13Na2CO331005.52AGL (30%) / 1-71.7332.81BuOH (70%)14K2CO331005.52AGL (30%) / 1-59.1762.23BuOH (70%)15Sc2CO331005.52AGL (30%) / 1-33.8164.46BuOH (70%)16Li2CO311505.52AGL (30%) / 1-62.9182.62BuOH (70%)17Na2CO311505...
examples 20-23
[0060]
Reaction of α-Angelica Lactone (AGL) with Cyclohexanol(CyHxOH) to Produce Cyclohexyl Levulinate (CyHxLV)N2AGLCyHxLVExpt.BasicTimeTempPressureConversionSelectivityNo.Catalyst(hrs)(° C.)(MPag)Feedstock(%)(%)20Li2CO311505.52AGL37.5638.03(30%) / CyHxOH(70%)21Na2CO311505.52AGL79.8114.82(30%) / CyHxOH(70%)22K2CO311505.52AGL94.072.32(30%) / CyHxOH(70%)23Cs2CO311505.52AGL80.6010.28(30%) / CyHxOH(70%)
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| cetane number | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com