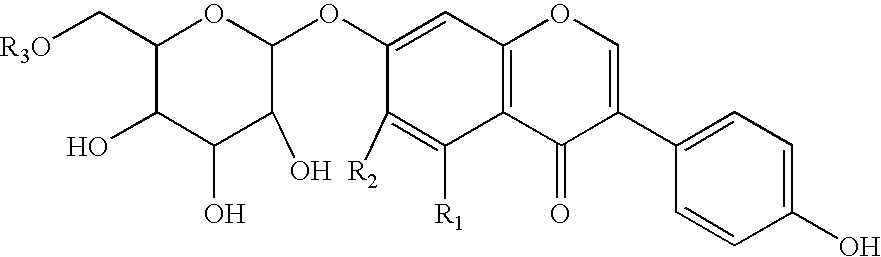
Soluble isoflavone compositions
a technology of isoflavone and composition, applied in the field of isoflavone composition, can solve the problems of increasing the risk of breast cancer and other complications, unable to increase the intake of isoflavone, and accessing this abundant diversity of compounds, etc., to achieve the effect of improving the aqueous solubility of isoflavone compounds, improving the solubility, and improving the taste characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of an Aqueous Plant Extract
Preparation 1: Aqueous Plant Extract from Soy Germ Flour
[0058] Hexane Extraction: A product containing greater than 80% soy germ was ground to yield soy flour. Soy germ products are well known in the art—see, e.g., U.S. Pat. No. 5,952,230 and PCT Publication No. WO 96 / 10341. Soy germ flour 20 kg was extracted with 53.5 kg of boiling hexane for 5 hours with continuous agitation. The extraction slurry was basket-centrifuged at 50° C. to separate the solids from re-claimed hexane to yield a total of 18.6 kg of defatted soybean meal. This defatted soybean meal was air desolventized at room temperature (21-22° C.) for 24 h.
[0059] Ethanol extraction and concentration: The desolventized meal was extracted with 290 kg of ethanol / water (80 / 20, v / v) at 59-62° C. for 15 hours with continuous agitation. The solids were once again isolated by centrifugation. Centrifugation was carried out at 50° C. to yield approximately 17.4 kg of solids and approximat...
example 2
Isolation of Isoflavones from Aqueous Plant Extracts
[0061] The aqueous plant extract from Preparation 1 (100 mL) was adjusted to pH 11.2 using 6 N NaOH. The aqueous phase was then extracted with 1-butanol (100 mL) and the layers separated. The pH of the aqueous phase was adjusted to 6.8 using concentrated HCl and was re-extracted with 1-butanol (60 mL) to yield a second butanol extract. The first butanol extract upon concentration under reduced pressure yielded 1.28 g of yellow solid (3.37% isoflavones), and the second butanol extract upon likewise treatment yielded 0.55 g of pale yellow solid (purity 37.78%, recovery: 56.9%).
[0062] In a separate experiment, the aqueous plant extract from Preparation 1 (100 mL) was adjusted to pH 11.8 using 6 N NaOH and extracted with ethyl acetate (100 mL) and the layers separated to yield a first organic extract. The pH of the aqueous phase was adjusted to 6.7 using concentrated HCl and it was extracted with ethyl acetate (100 mL) to yield a sec...
example 3
Preparation of Isoflavone Compositions from Organic Plant Extracts by Butanol Addition
[0063] Through stirring, 1.08 g of an isoflavone sample (produced as described in Example 2) was dissolved in 1000 ml of 94% butanol / water. PEG 200 (0.05 g) was added with continued stirring. The mixture was concentrated through rotary evaporation to a solid, then redissolved in hot water and concentrated to a solid again through rotary evaporation. The same procedure was repeated, independently, with 0.10 g of PEG 200, PEG 400, PEG 600, and PEG 1000.
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittance | aaaaa | aaaaa |
| transmittance | aaaaa | aaaaa |
| transmittance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com