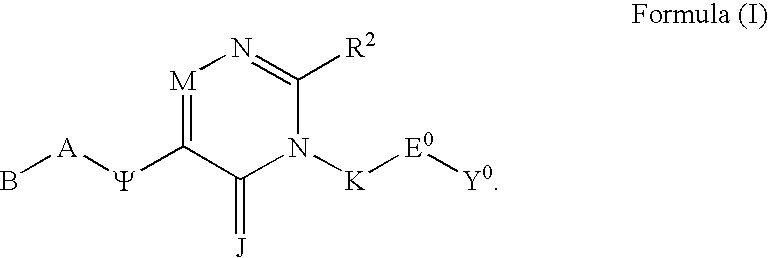
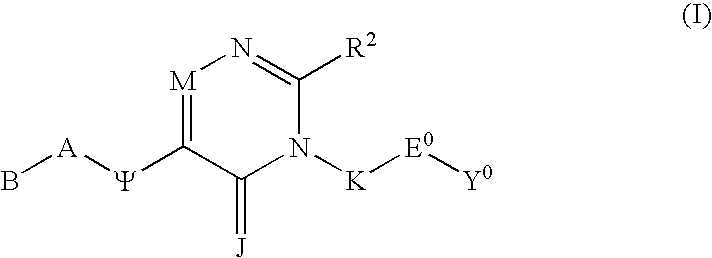
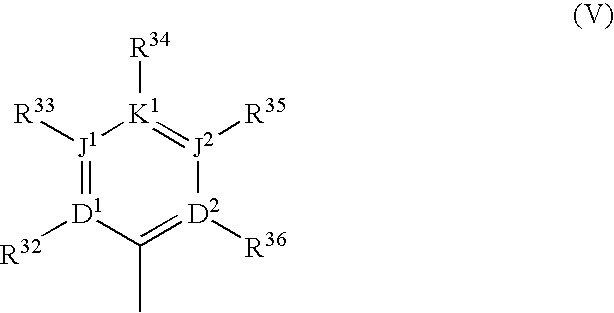
Substituted polycyclic aryl and heteroaryl pyrimidinones useful for selective inhibition of the coagulation cascade
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(EX-1A) A solution of ethyl benzimidate hydrochloride (92.25 g, 496.9 mmol) in 300.0 mL dry methanol (1.68 M) was cooled to ca 0° C. and added a solution of aminoacetaldehyde dimethyl acetal (73.10 mL, 670.9 mmol) in dry methanol (75.0 mL, 9.0 M) drop wise at such a rate the temperature was kept below 5° C. The resulting solution was allowed to stir for 3 days with the temperature being maintained below 5° C. The reaction mixture was then concentrated under reduced pressure to give a yellow oil. The residue was dissolved in 1 N NaOH (750 mL) and extracted with dichloromethane (4×250 mL). The organic solutions were combined, dried (MgSO4), and concentrated to give 108.13 g crude N-(2,2-dimethoxyethyl)benzamidine as a yellow oil. The crude N-(2,2-dimethoxyethyl)benzamidine (108.13 g, 519.2 mmol) in dry methanol (125.0 mL, 4.2 M) was added dimethyl methoxymethylenemalonate (94.13 g, 540.5 mmol) in one portion at room temperature. The resulting mixture was heated to approximately 100° ...
example 2
By following the method of Example 1, the title compound was prepared: 1H NMR (300 MHz, d-DMSO) δ 9.40-9.33 (m, 4H), 8.86 (s, 1H), 7.82 (s, 2H), 7.72-7.37 (m, 15H), 4.65-4.59 (m, 4H), 4.41-4.40 (m, 2H); HRMS (EI) calcd for C27H27N6O4S 531.1815, found 531.1794.
example 3
(EX-3A) A solution of [5-amino-2-phenyl-6-oxo-1,6-dihydro-1-pyrimidinyl]acetic acid t-butyl ester (EX-1F) (613.7 mg, 2.037 mmol) in 7.0 mL tetrahydrofuran and dichloromethane (1:1, 0.3 M) was added 25.0 mL acetic acid and phenylacetaldehyde (0.475 mL, 4.060 mmol). The solution was cooled to 0° C. in an ice bath and added sodium triacetoxyborohydride (1.9131 g, 9.027 mmol) in one portion. After stirring for 5 minutes, the ice bath was removed and the reaction mixture was allowed to stir at room temperature for 2 hours. The reaction was quenched by the addition of 1 N NaOH (5 mL), and the mixture was stirred for 5 minutes. The reaction mixture was diluted 0.5 N NaOH (100 mL). The aqueous solution was extracted with ethyl acetate (3×25 mL). The combined organic solutions were washed with 0.5 N NaOH (1×25 mL) and brine (1×25 mL). The solution was dried (MgSO4), filtered and concentrated under reduced pressure. Purification by MPLC (25% ethyl acetate / hexanes) afforded EX-3A as a yellow ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com