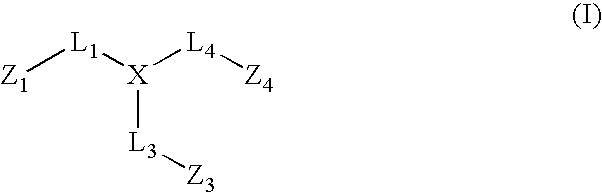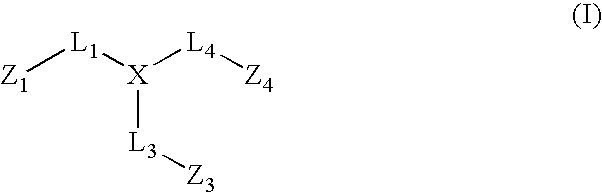Substituted 5-membered polycyclic compounds useful for selective inhibition of the coagulation cascade
a technology of coagulation cascade and polycyclic compounds, which is applied in the direction of drug compositions, biocides, extracellular fluid disorders, etc., can solve the problems of ischemic necrosis of tissue supplied by the artery, myocardial infarction or heart attack, and reduce blood flow, so as to prevent and treat thrombotic conditions in mammals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0049] One aspect of the invention embraces compounds that correspond to formula (I)
wherein:
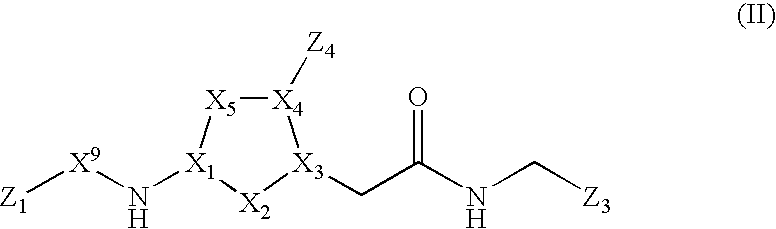
[0050] X comprises a 5-membered heterocyclic or carbocyclic ring, the ring atoms being X1, X2, X3, X4, and X5, wherein X1, X3, and X4, are independently carbon or nitrogen and X2 and X5 are independently carbon, nitrogen, oxygen or sulfur;
[0051] L1, L3 and L4 are linkages through which Z1, Z3, and Z4, respectively, are covalently bonded to different ring atoms of the 5-membered heterocyclic or carbocyclic ring of X, wherein Z1 is covalently bonded to X1, Z3 is covalently bonded to X3, and Z4 is covalently bonded to X4, each of L1, L3 and L4 independently being a covalent bond or comprising one or more atoms through which Z1, Z3, and Z4 are covalently bonded to X1, X3 and X4, respectively;
[0052] Z1 is hydrocarbyl or substituted hydrocarbyl;
[0053] Z3 comprises a 5- or 6-membered heterocyclic or aromatic ring substituted with an amidine group, the ring atoms of the 5- or 6-membered hetero...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| aggregation | aaaaa | aaaaa |
| polar | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com