Tumor homing molecules, conjugates derived therefrom, and methods of using same
a tumor and homing technology, applied in the field of tumor homing molecules and conjugates, can solve the problems of relative lack of agents that can selectively target the cancer, the ability to treat patients presenting with more advanced disease only minimally, and the inability to keep up with the diagnostic process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
In Vivo Panning
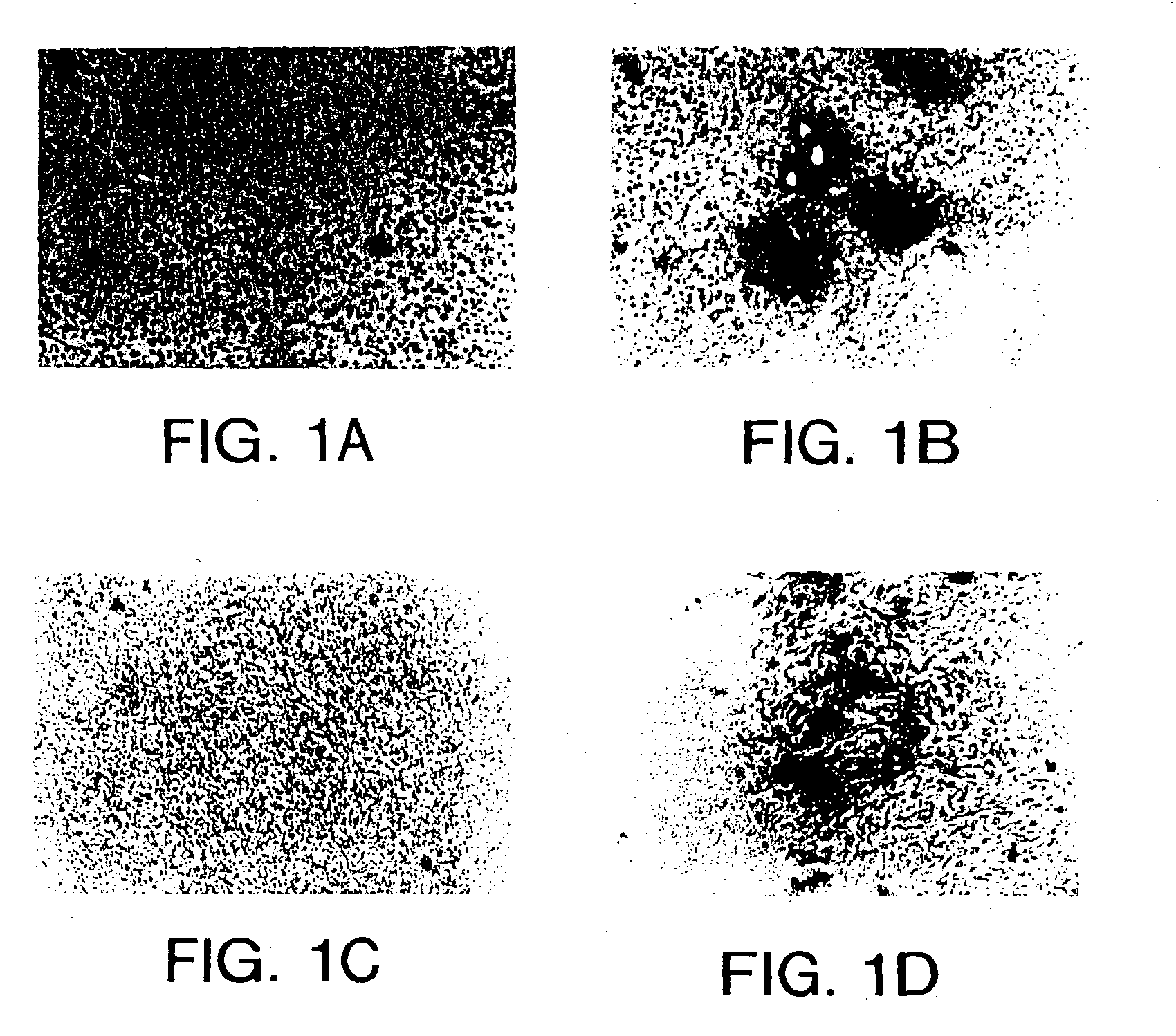
[0147] This example demonstrates methods for preparing a phage library and screening the library using in vivo panning to identify phage expressing peptides that home to a tumor.
[0148] A. Preparation of Phage Libraries
[0149] Phage display libraries were constructed using the fuse 5 vector as described by Koivunen et al. (supra, 1995; Koivunen et al., supra, 1994b). Libraries encoding peptides designated CX.sub.5C (SEQ ID NO: 9), CX.sub.6C (SEQ ID NO: 10), CX.sub.7C (SEQ ID NO: 11) and CX.sub.3CX.sub.3CX.sub.3C (SEQ ID NO: 12) were prepared, where "C" indicates cysteine and "X.sub.N" indicates the given number of individually selected amino acids. These libraries can display cyclic peptides when at least two cysteine residues are present in the peptide. In addition, a library that did not contain defined cysteine residues also was constructed. Such a library results in the production primarily of linear peptides, although cyclic peptides also can occur due to random pr...
example ii
Identification of Tumor Homing Peptides By In Vivo Panning against a Breast Tumor
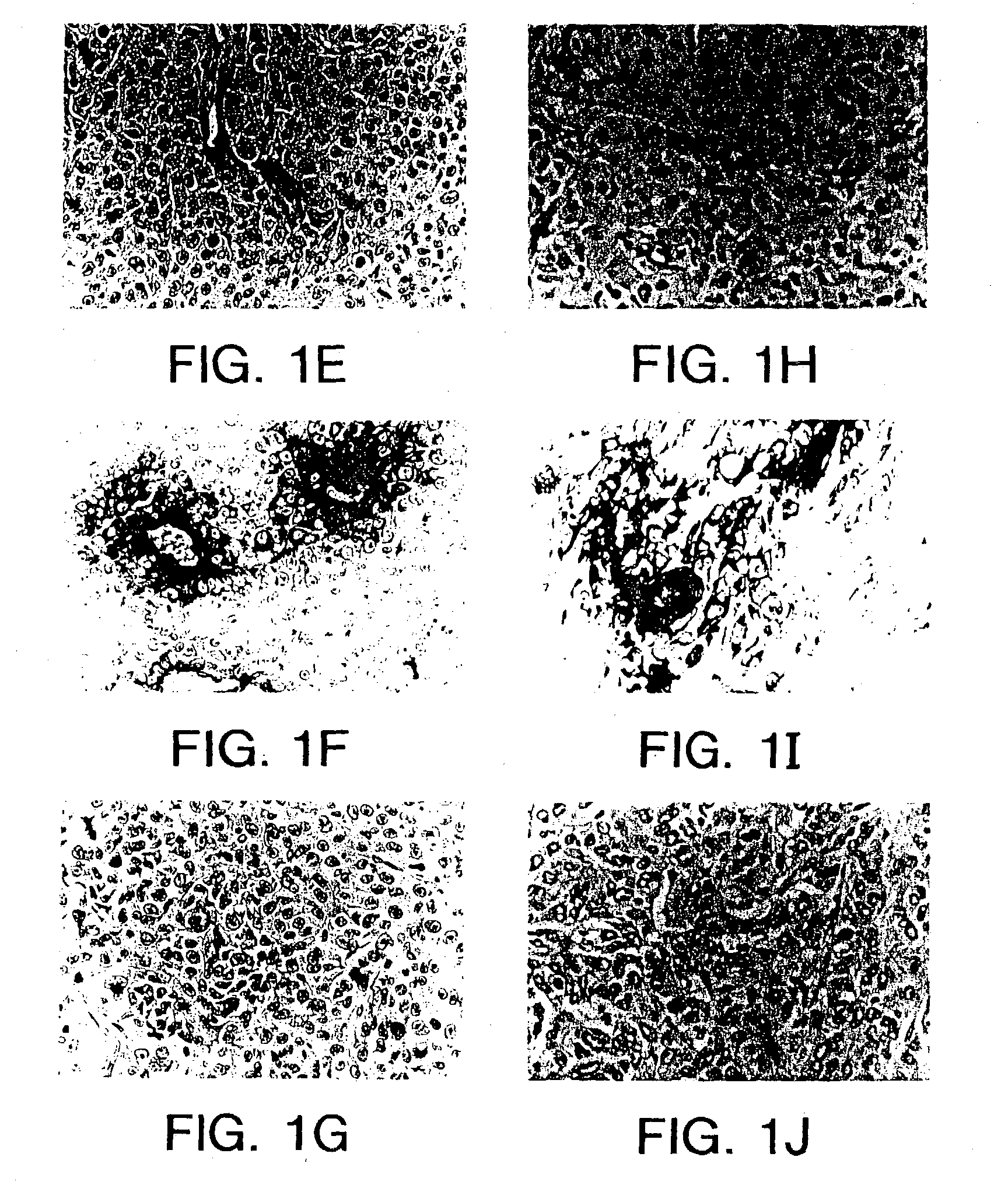
[0158] This example demonstrates that in vivo panning can be performed against a breast tumor to identify tumor homing peptides that home to various tumors.
[0159] Human 435 breast carcinoma cells (Price et al., Cancer Res. 50:717-721 (1990)) were inoculated into the mammary fat pad of nude mice. When the tumors attained a diameter of about 1 cm, either a phage targeting experiment was performed, in which phage expressing a specific peptide were administered to the tumor bearing mouse, or in vivo panning was performed.
[0160] The breast tumor bearing mice were injected with 1.times.10.sup.9 phage expressing a library of CX.sub.3CX.sub.3CX.sub.3C (SEQ ID NO: 12) peptides, where X.sub.3 indicates three groups of independently selected, random amino acids. The phage were allowed to circulate for 4 min, then the mice were anesthetized, snap frozen in liquid nitrogen while under anesthesia, and the tumor was r...
example iii
In Vivo Targeting of a Phage Expressing an an RGD Peptode to a Tumor
[0162] Human 435 breast carcinoma cells were inoculated into the mammary fat pad of nude mice. When the tumors attained a diameter of about 1 cm, phage expressing a specific RGD-containing peptide were administered to the tumor bearing mouse. Similar results
1TABLE 1 PEPTIDES FROM PHAGE RECOVERED FROM HUMAN BREAST CANCER CGRECPRLCQSSC (2*) CNGRCVSGCAGRC (3) CGEACGGQCALPC (20) IWSGYGVYW (21) PSCAYMCIT (22) WESLYFPRE (23) SKVLYYNWE (24) CGLMCQGACFDVC (25) CERACRNLCREGC (26) CPRGCLAVCVSQC (27) CKVCNGRCCG (28) CEMCNGRCMG (29) CPLCNGRCAL (30) CPTCNGRCVR (31) CGVCNGRCGL (32) CEQCNGRCGQ (33) CRNCNGRCEG (34) CVLCNGRCWS (35) CVTCNGRCRV (36) CTECNGRCQL (37) CRTCNGRCLE (38) CETCNGRCVG (39) CAVCNGRCGF (40) CRDLNGRKVM (41) CSCCNGRCGD (42) CWGCNGRCRM (43) CPLCNGRCAR (44) CKSCNGRCLA (45) CVPCNGRCHE (46) CQSCNGRCVR (47) CRTCNGRCQV (48) CVQCNGRCAL (49) CRCCNGRCSP (50) CASNNGRVVL (51) CGRCNGRCLL (52) CWLCNGRCGR (53) CSKCNGRCGH (54) CV...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com