Process for the preparation of cefuroxime sodium
a technology of cefuroxime and sodium, which is applied in the field of process for the preparation of cefuroxime sodium, can solve the problems of complex process, low purity, and inacceptable color of the product obtained from this patent, and achieve the effect of improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
(2)
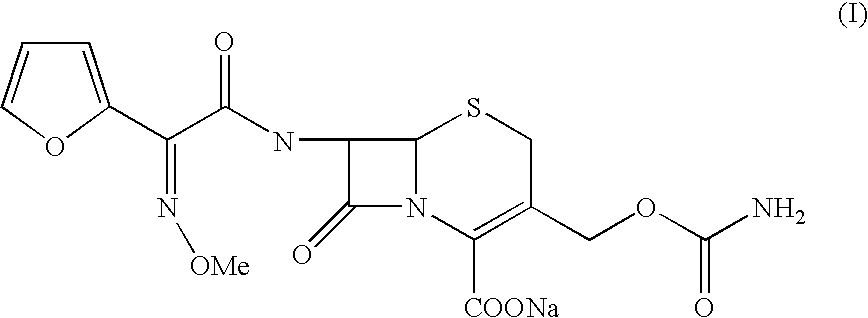
[0042] Preparation of (6R,7R)-3-carbamoyloxymethyl-7-[Z2-(fur-2-yl)-2-meth-oxy iminoacetamido]-ceph-3-em-4-carboxylic Acid Sodium (Cefuroxime Sodium):
[0043] (6R,7R)-3-carbamoyloxymethyl-7-[Z-2-(fur-2-yl)-2-methoxyimino acetamido]-ceph-3-em-4-carboxylic acid (100 gm) was dissolved in a mixture of acetone (650 ml) / water (800 ml) at 25.degree. C. Activated carbon was added and stirred for 15 minutes at 25.degree. C. The carbon was filtered and washed the bed with acetone / water. The solution was then passed through series of micron filters in a sterile area. The solution was warmed to 35.degree. C. A mixture of sodium lactate solution (23 gm) (60% solution in water) and sodium acetate (16.5 gm) in methanol (450 ml) was added to the reaction mixture slowly at 35.degree. C. and stirred for 30 minutes. The product obtained was filtered and washed with methanol (200 ml) followed by acetone. The product was dried under vacuum to get sterile cefuroxime sodium (98 gm) in pure form.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com