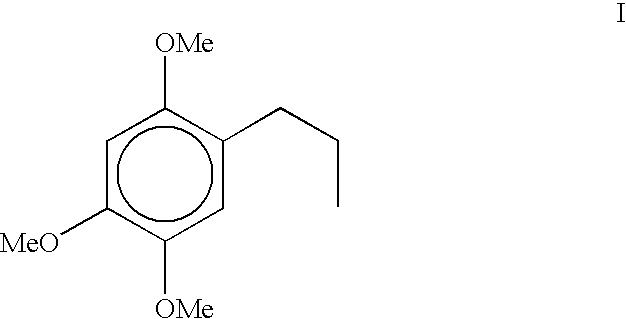
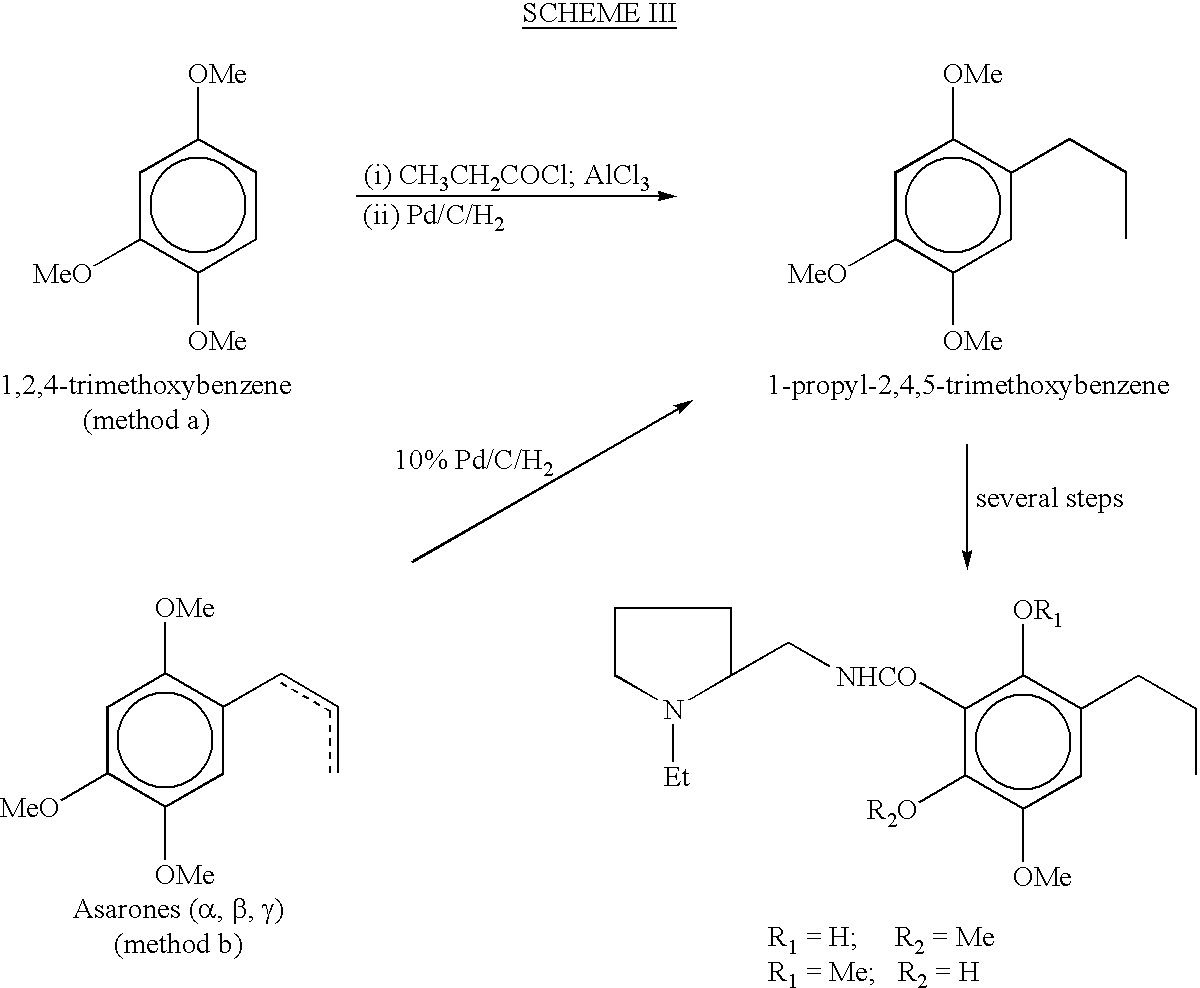
Process for the preparation of 1-Propyl-2, 4, 5- trimethoxybenzene from toxic beta-asarone of Acorus calamus or from crude calamus oil containing beta-asarone
a technology of acorus calamus and beta-asarone, which is applied in the field of process for the preparation of 1propyl2, 4, 5trimethoxybenzene, can solve the problems of affecting the medicinal potential of acorus calamus, affecting the use of acorus calamus, and affecting the ability of acorus calamus to achieve the effect of reducing the high percentage (70 to 90%) of asar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0065] Plant Material and Chemicals: The plant material was collected in March-April 1999 from Palampur (H.P.) and was confirmed as `Acorus calamus Linn` by comparison with the specimen (1HBT no. 1066) kept in the herbarium of our Institute. The hydrodistillation of rhizomes of Acorus calamus gave pungent smelling oil in 1.7% yield (w / w) with a presence of 81-85% .beta.-asarone (by GC). .alpha.-asarone is procured from Sigma chemical s (U.S.A) and is used as an authentic sample.
[0066] Isolation of .beta.-asarone from Acorus calamus: The pale yellow calamus oil (17.00 g) was chromatographed over silica gel using hexane as eluent to remove unwanted non-polar compounds. Subsequent elution with hexane-benzene mixture with increasing proportion of benzene gave a pure liquid 13.94 g in 82% yield (w / w) with R.sub.f 0.63 on silica gel TLC plate (hexane:benzene / toluene:ethylacetate:: 1:1:0.1) whose electrospray (ES)-mass spectrum gave a molecular ion at m / e 208 (M+, 100), 193 (M.sup.+-Me, 46...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com