Novel statin pharmaceutical compositions and related methods of treatment
A composition and drug technology, applied in the direction of pharmaceutical formulations, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0186] Pravastatin calcium salt
[0187] To a solution of pravastatin Na salt (1.470 g; 3.292 mmol) in water (15.0 mL) was added a solution of calcium acetate (268 mg; 1.70 mmol) in water (5.0 mL). The resulting solution was concentrated (water was evaporated by a stream of nitrogen) to about 15 mL and cooled to 0°C. The precipitated white solid was collected by filtration. The filtrate was cooled to 0°C again, and further precipitation occurred. After filtration, the solids were combined and dried in a water extractor. The obtained solid was determined to be pravastatin calcium salt. The resulting salt was a 2:1 pravastatin-calcium salt.
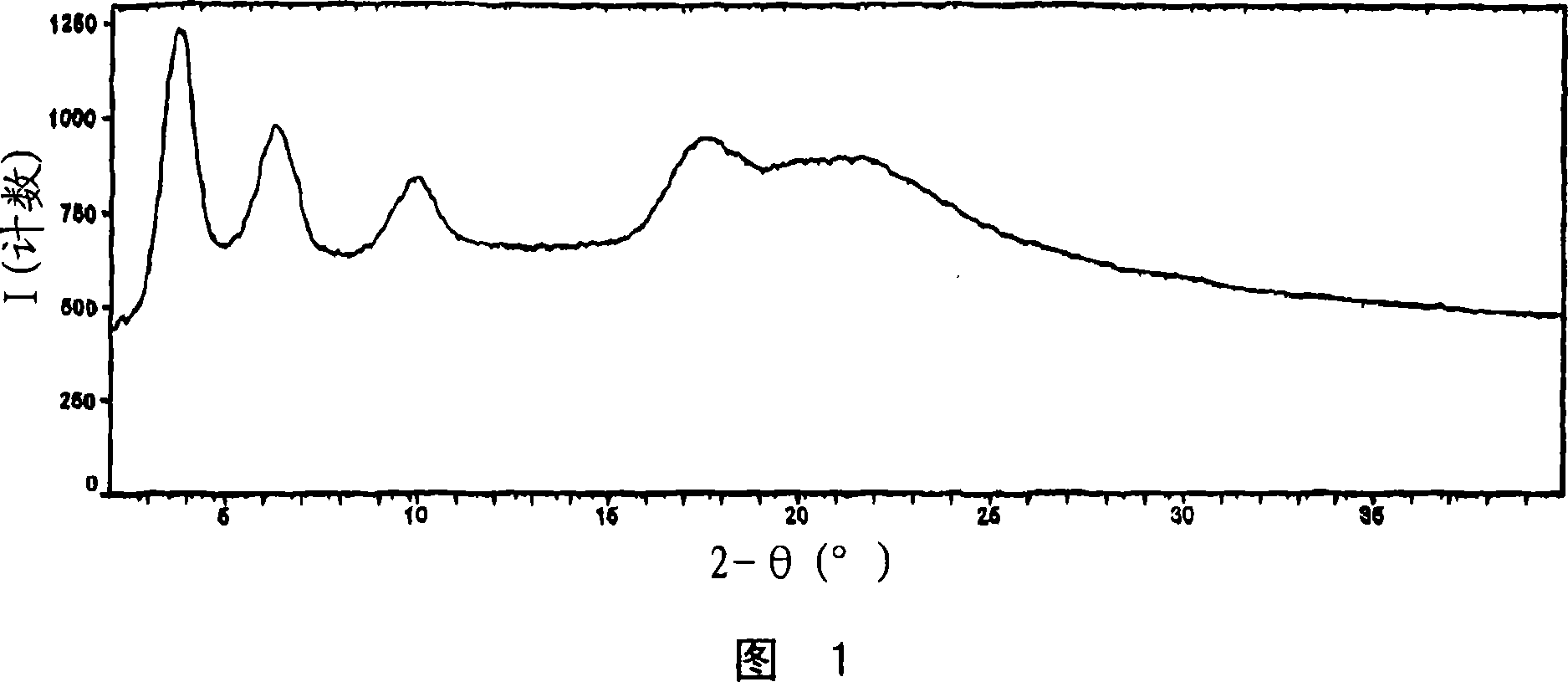
[0188] Figure 1 shows the PXRD diffraction pattern of pravastatin calcium salt (Bruker, data collection). Pravastatin calcium salt can be characterized by any one, any two, any three or any four or more PXRD peaks in Figure 1 . From this PXRD diffraction pattern, it appears that pravastatin calcium salt is weakly crystalline.
[0189...
Embodiment 2
[0195] 26 Week Solubility Data of Pravastatin Salt in E463808
[0196] Several salts of pravastatin were suspended in E463808 omega-3 oil and placed in capped glass vials or hermetically sealed gelatin capsules. The soft capsules were used at 25°C and the glass vials at 25, 40 and 60°C. Suspensions of salt in oil were periodically measured for 26 weeks. Degradation of pravastatin salts was measured using HPLC.
[0197] Figure 6 shows stability data at 25°C for vials and gelatin capsules (soft capsules). The calcium salt of pravastatin showed a significantly lower percentage of impurities over time than either the sodium salt in the softgel or the potassium salt in the vial. The calcium salt in the vial showed the least degradation of all the salts in softgels or vials after 26 weeks at 25°C.
[0198] Figure 7 shows the stability of pravastatin salt in capped vials at 40 and 60°C. Calcium salts again showed significantly lower degradation than sodium or potassium salts at ...
Embodiment 3
[0200] fluvastatin calcium salt
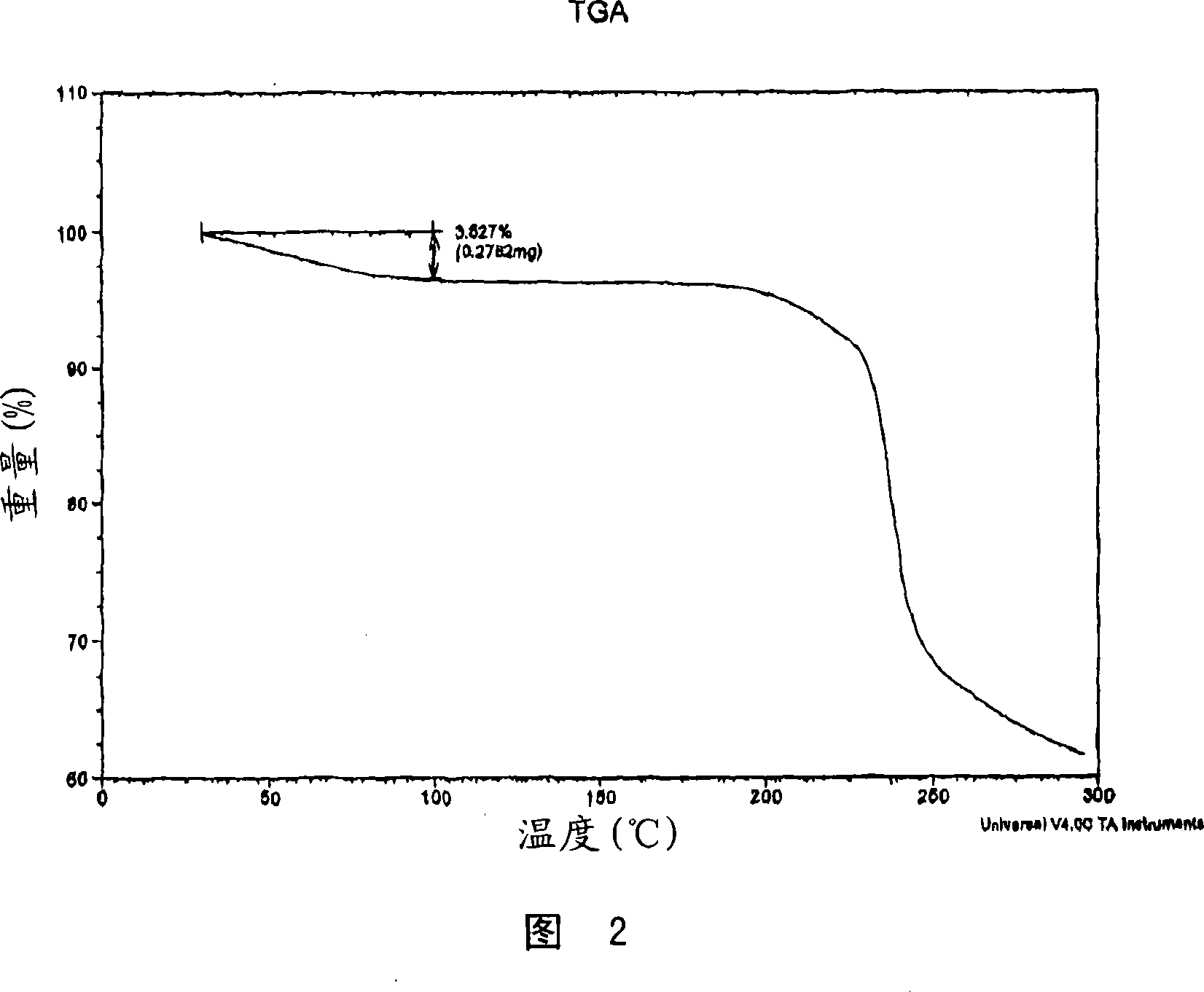
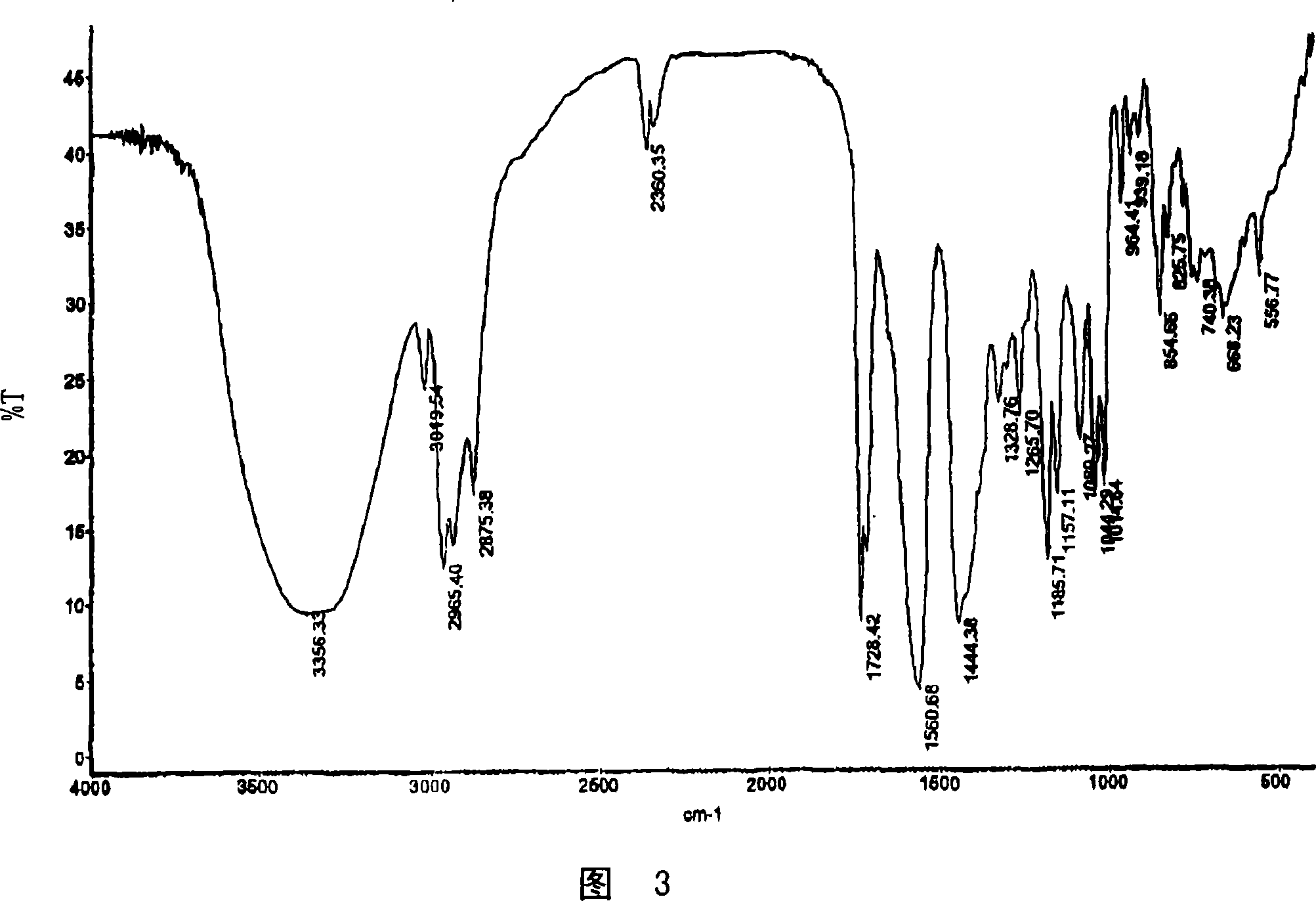
[0201] Dissolve 505.9 mg (1.167 mmol) of fluvastatin Na salt in 15 mL of water. Dissolve 94.2 mg (0.595 mmol) of calcium acetate in 2 mL of water. Immediately after the addition of the calcium acetate solution to the fluvastatin Na solution, a precipitate formed. The solid was collected by filtration, first dried in a vacuum oven at 65°C for 0.5 h, and then placed under a nitrogen stream at room temperature overnight. The dried solids were ground in a mortar and pestle prior to characterization. The resulting solid was characterized using PXRD, DSC, TGA, Raman and IR spectroscopy and was determined to be the calcium salt of fluvastatin. The resulting salt was a 2:1 fluvastatin-calcium salt.
[0202] Solubility measurements of fluvastatin sodium and calcium salts were performed in water at 23°C. Solubility was measured gravimetrically in deionized water. 5.5 mg of fluvastatin sodium salt was dissolved in about 130-150 microliters of water...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Water solubility | aaaaa | aaaaa |
| Water solubility | aaaaa | aaaaa |
| Water solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com