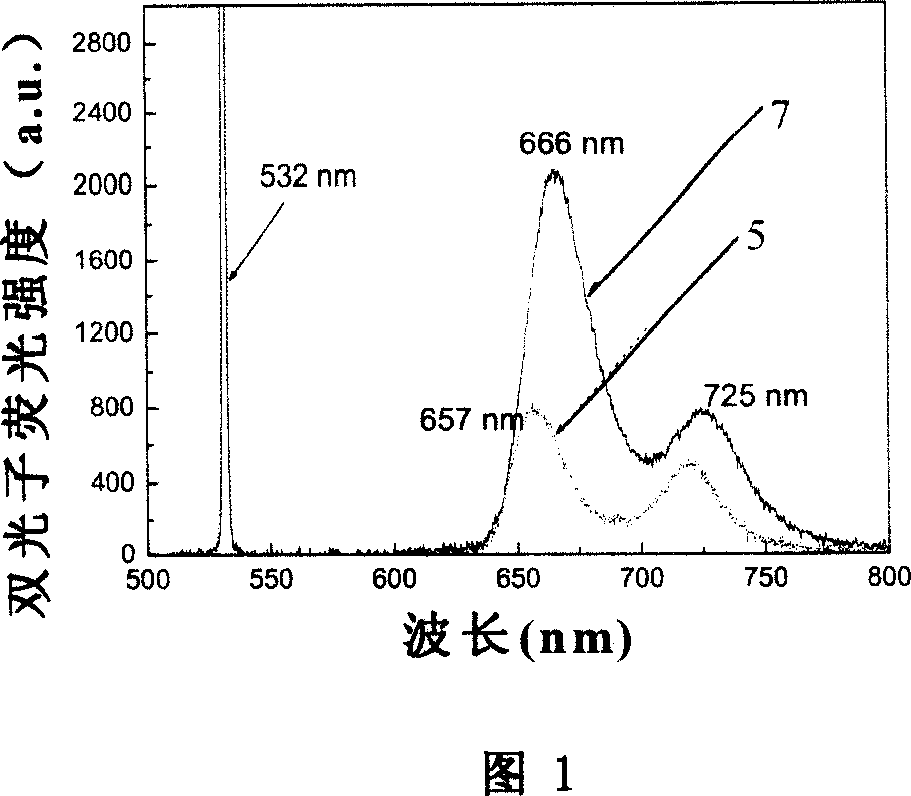
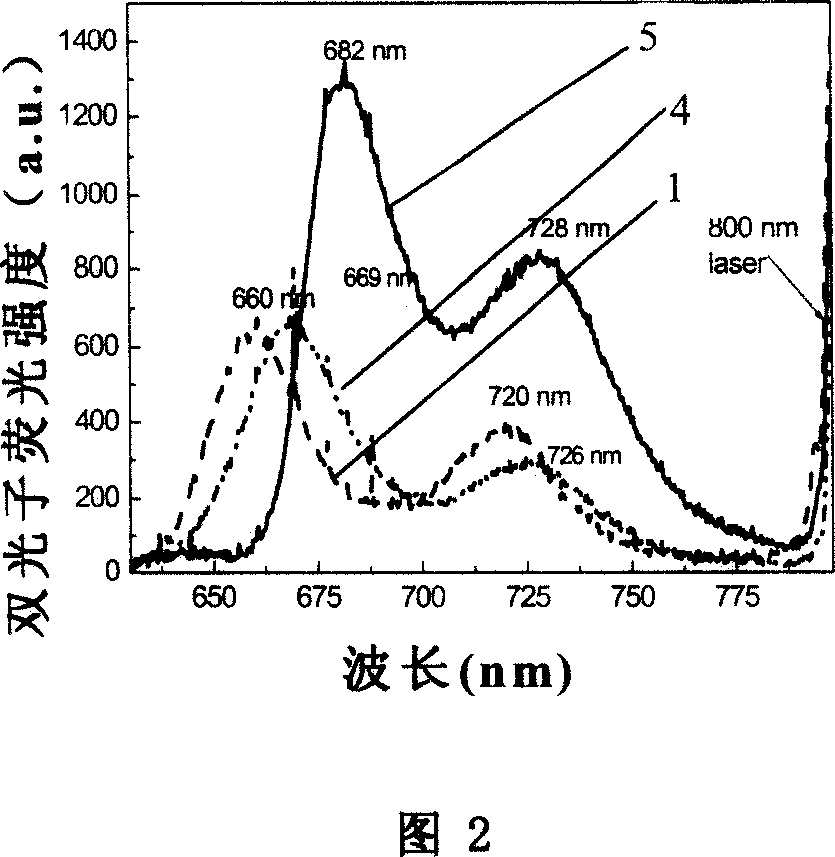
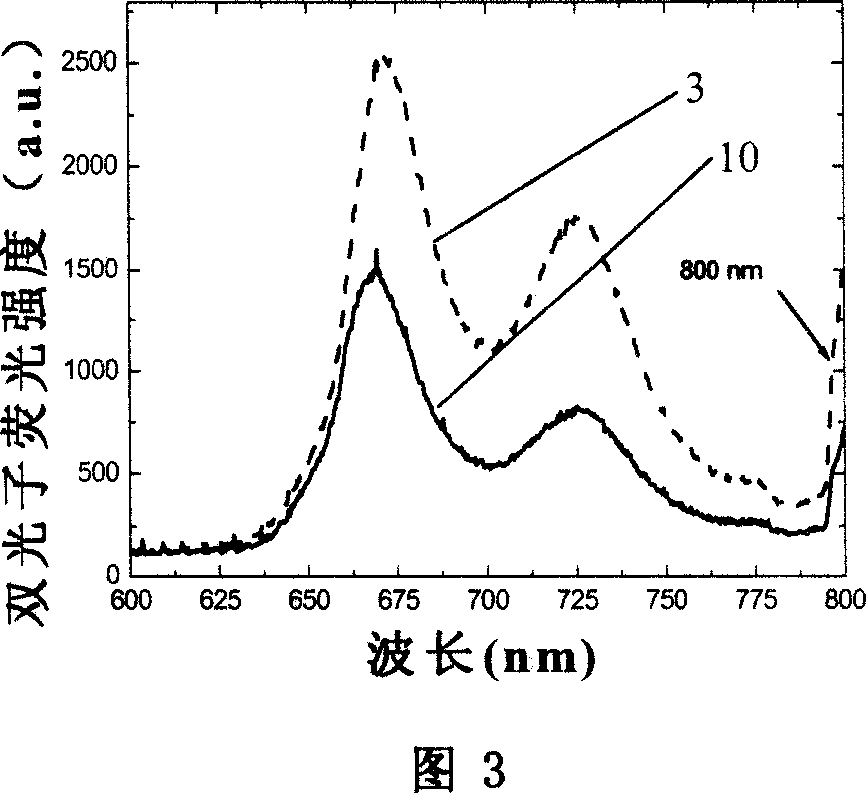
Branchy porphyrin molecule with the characteristics of intramolecular energy transfer and two-photon absorption
A technology of two-photon absorption and energy transfer, applied in the field of porphyrin derivatives, can solve the problems of unfavorable photosensitizer absorption, weak linear absorption, affecting practical application, etc., and achieve the effect of excellent optical performance and strong two-photon absorption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Synthesis of 5,10,15,20-tetrakis-[4-E-(diphenylamino)styryl)]-tetraphenylporphyrin (abbreviated as TPPX4).
[0043] (1) Add 1.86g (0.01mol) p-bromobenzaldehyde and 45mL propionic acid into a 250mL three-necked flask, reflux at 140°C, dissolve 0.7g freshly distilled pyrrole in 15mL propionic acid, slowly drop into the flask, and react 80min, cooled to room temperature, left standing, suction filtered, washed with propionic acid, dried to obtain 0.55g purple powder: 5,10,15,20-tetra-(4-bromo)-tetraphenylporphyrin (abbreviated as TPP- Br4). Yield 23.70%. Mp>300℃. 1 H NMR (CDCl 3 ;400MHz; Me 4 Si): δ, ppm-2.87(s, 2H, NH), 7.89-7.91(d, 8H, J=8.4Hz), 8.06-8.08(d, 8H, J=8.4Hz).
[0044] (2) In a closed device, 0.28g (0.31mmol) TPP-Br4, 0.5g (1.85mmol) 4-E-(diphenylamino) styrene, 0.093g (0.31mmol) o-methyltriphenyl Add phosphine into the reaction flask, inject nitrobenzene (15mL) and tributylamine (15mL) and a small amount of catalyst, and react under reflux a...
Embodiment 2
[0045]Example 2: 5,10,15,20-four-{two-[4-E-(diphenylamino)styrene]-N-4-E-(diphenylamino)styrene}tetraphenylporphyrin (TPPX12 for short) synthesis.
[0046] The method is similar to Example 1, only need to change 4-E-(diphenylamino)styrene in step 2 into 4,4'-bis-(4-E-(diphenylamino)styrene according to the obtained compound Base-N-4-E-(diphenylamino)styrene. Use chloroform / petroleum ether (1:1 v / v) as the mobile phase to pass through the silica gel column, take the second phase red component, and obtain the product purple Solid, 4.1% yield.
Embodiment 3
[0047] Example 3: Synthesis of 5,10,15,20-tetra-[4-E-(N-carbazolyl)styryl]-tetraphenylporphyrin (TPPCz4 for short).
[0048] Similar to Example 1, it is only necessary to change 4-E-(diphenylamino)styrene in step 2 to 4-E-N-carbazole styrene according to the obtained compound. Yield 8.3%. Mass spectrum (MALDI-TOF-TOF): 1685 (M + ), 1417 (fragmentation peak, loss of 1 4-N-carbazole styryl group), 1150 (fragmentation peak, loss of 2 4-N-carbazole styryl groups). 1 H NMR (CDCl 3 ;400MHz; Me 4 Si): δ (ppm), -2.540 (s, 2H, -NH porphyrin), 7.327-7.376 (q, 4H, J = 6.5Hz, double bond), 7.463-7.513 (q, 4H, J = 6.6Hz, double bond), 7.534, 7.560, (d, 8H, J=10.4Hz, phenyl), 7.794, 7.874 (d, 8H, J=8.0Hz, phenyl), 7.956-8.037 (m, 16H, J=8.1Hz, phenyl ), 8.291-8.387 (m, 16H, phenyl), 8.678-8.587 (m, 16H, phenyl), 8.867 (s, 8H, pyrrole, β-H protons).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com