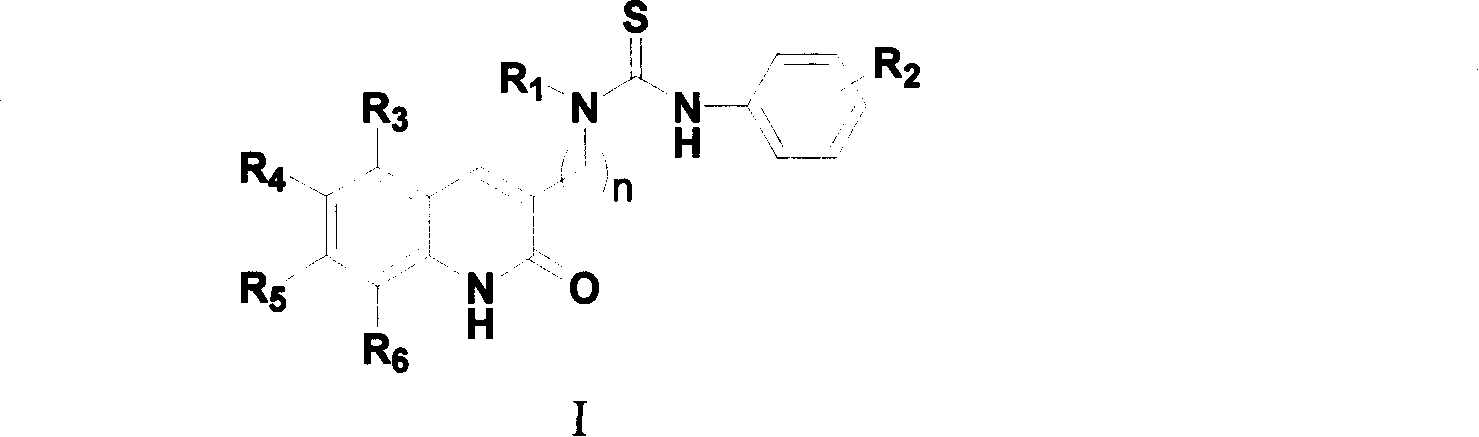
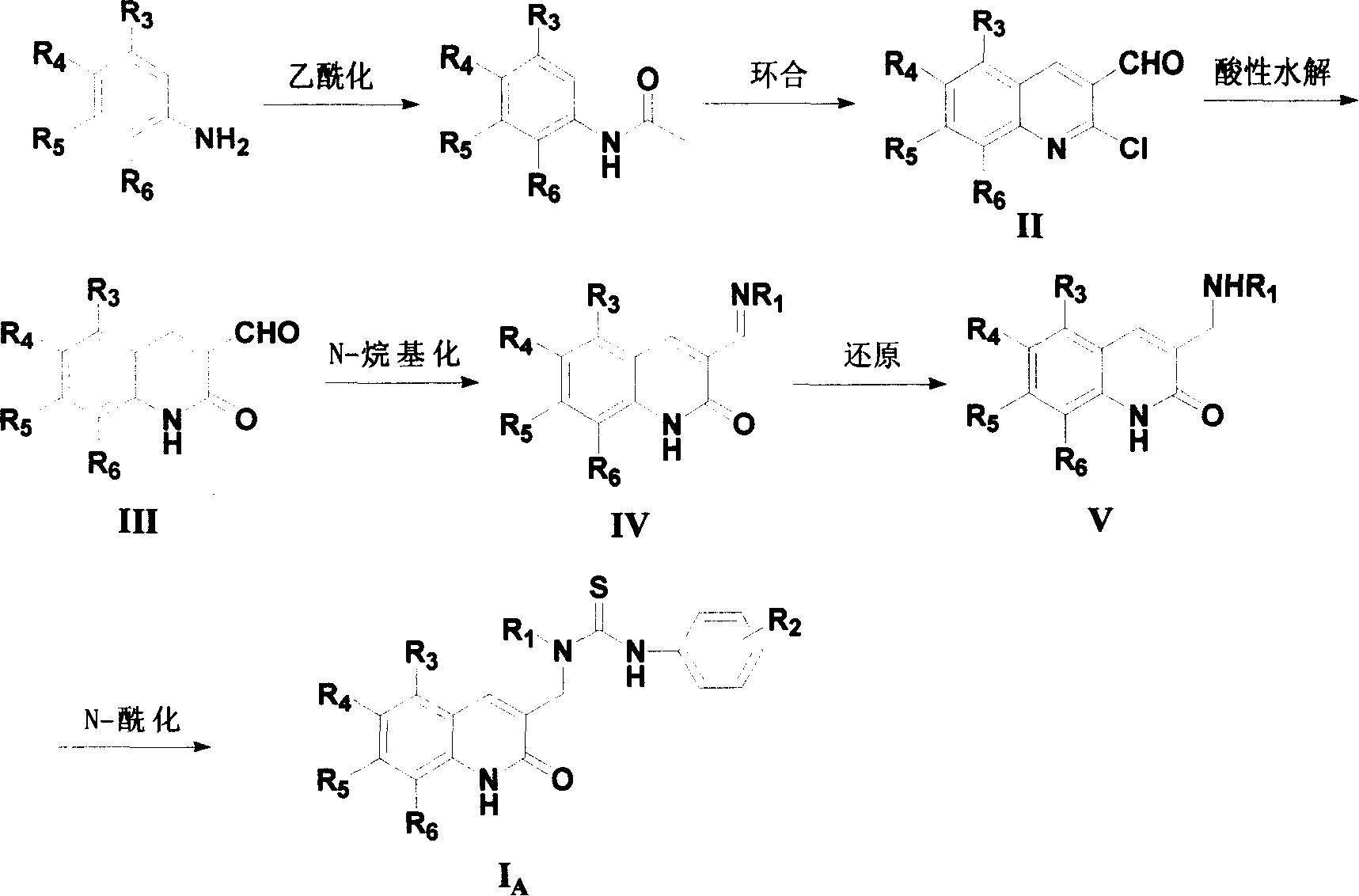
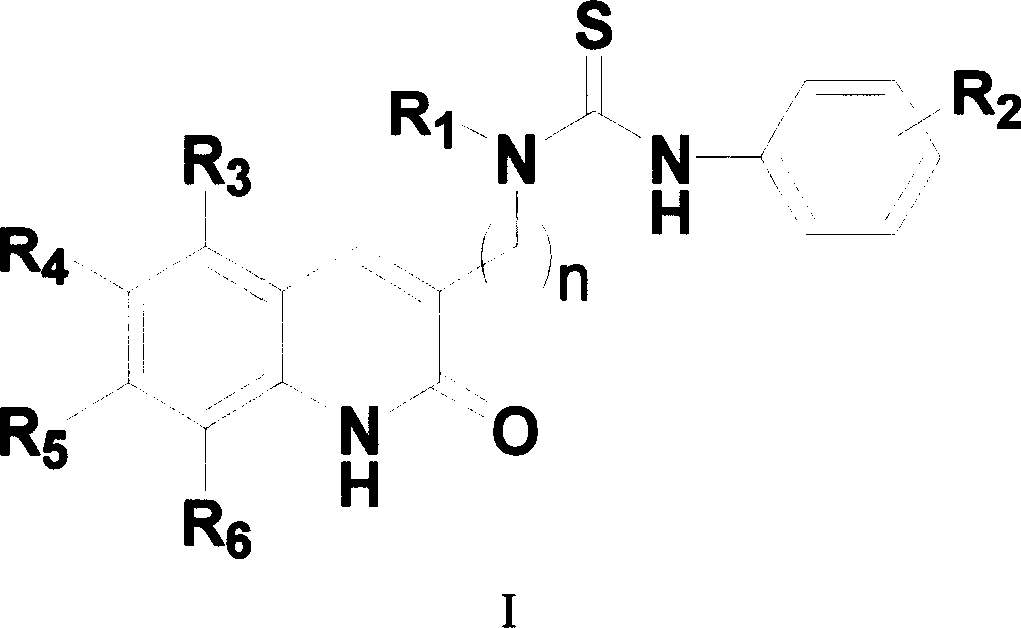
Compound in category of dihydro quinolines, preparation method, and composition of medication
A compound and mixture technology, applied in the fields of medicinal chemistry and pharmacotherapy, can solve the problems of unknown toxicity of 4-anilinoquinazoline compounds, and achieve the effects of abundant raw materials, mild reaction conditions and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 2-Chloro-quinoline-3-carbaldehyde (II-1)
[0052] Add 4.6 g of DMF into a 50 ml round-bottomed flask, and slowly add 26.8 g of POCl dropwise under cooling and stirring in an ice bath 3 . After the dropwise addition, 3.4 g of N-acetanilide was added at one time, and after stirring for 5 minutes, the temperature was raised to 75° C. and refluxed for 16 hours. The reaction solution was poured into 150 ml of ice water, stirred for 30 minutes, a large amount of solids were precipitated, filtered with suction, washed with water, and dried to obtain 3.1 g of 2-chloro-quinoline-3-carbaldehyde as a yellow solid product, with a yield of 64.8%. Mp 144-146°C (lit. 148-149°C).
Embodiment 2
[0054] 2-oxo-1,2-dihydro-quinoline-3-carbaldehyde (III-1)
[0055] Put 3.1 g of 2-chloro-quinoline-3-carbaldehyde (II-1) into 55 ml of 4M hydrochloric acid, stir and reflux for 1 hour, cool to room temperature, a large amount of solid precipitated, suction filtered, washed with water, and dried to obtain a yellow solid product 2.6 grams, yield 92.8%. Mp 300°C. MS-EI 173(M), 144 (100%).
Embodiment 3
[0057] 3-[(Pyridine-3-methylimino)methyl]-1H-quinolin-2-one (IV-1)
[0058] Dissolve 0.5 g of 2-oxo-1,2-dihydro-quinoline-3-carbaldehyde and 0.32 g of 3-aminomethylpyridine in 60 ml of absolute ethanol, stir at reflux for 12 hours, and evaporate most of the The solvent was cooled to room temperature, pumped up, and washed with a small amount of absolute ethanol to obtain 0.6 g of light yellow solid with a yield of 78.9%. Mp 180-182°C; 1 H-NMR (400Hz, DMSO-d6) δ: 4.83 (2H, s), 7.21 (1H, t), 7.33 (1H, d), 7.39 (1H, m), 7.56 (1H, t), 7.76 (1H , d), 7.83 (1H, d), 8.49 (2H, m), 8.59 (1H, d), 8.73 (1H, t). MS-EI 262 (M-1, 100%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com