Novel phenolic acid compounds of tanshin polyphenolic acid N and application thereof
A kind of technology of salvianolic acid and compound, which is applied to new phenolic compound salvianolic acid N and its application field, and can solve problems such as no reports.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1. extraction method
[0023] Take 4 kg of the root of Danshen medicinal material, cut into decoction pieces, add 8 times the amount of water to soak for 0.5 hour, decoct and extract for 1 hour, filter, and the filtrate is set aside. The dregs were decocted with 6 times the amount of water to extract twice, each time for 0.5 hours, filtered, the filtrates were combined three times, concentrated under reduced pressure, and 0.8 kg of extract was obtained.
Embodiment 2
[0024] Embodiment 2. Purification method
[0025] Add 5 times the amount of water to the extract extract and heat to dissolve, slowly inject into the processed HP20 type, SP825 type macroporous adsorption resin column, first elute with water, discard; then elute with 50% ethanol, collect medicinal materials 3 Double the amount of 50% ethanol eluate, concentrate, and dry to obtain 170 g of total phenolic acids.
[0026] Heat and dissolve the total phenolic acids with water, slowly add to the Sephadex LH-20 column, first elute with water, then elute with 2 times column volume of 40% ethanol, discard; then use 60% of 2 times column volume Eluted with ethanol, the eluate was collected, concentrated, and purified by repeated column chromatography on an ODS column to obtain the new compound salvianolic acid N.
Embodiment 3
[0027] Embodiment 3. Structural identification:
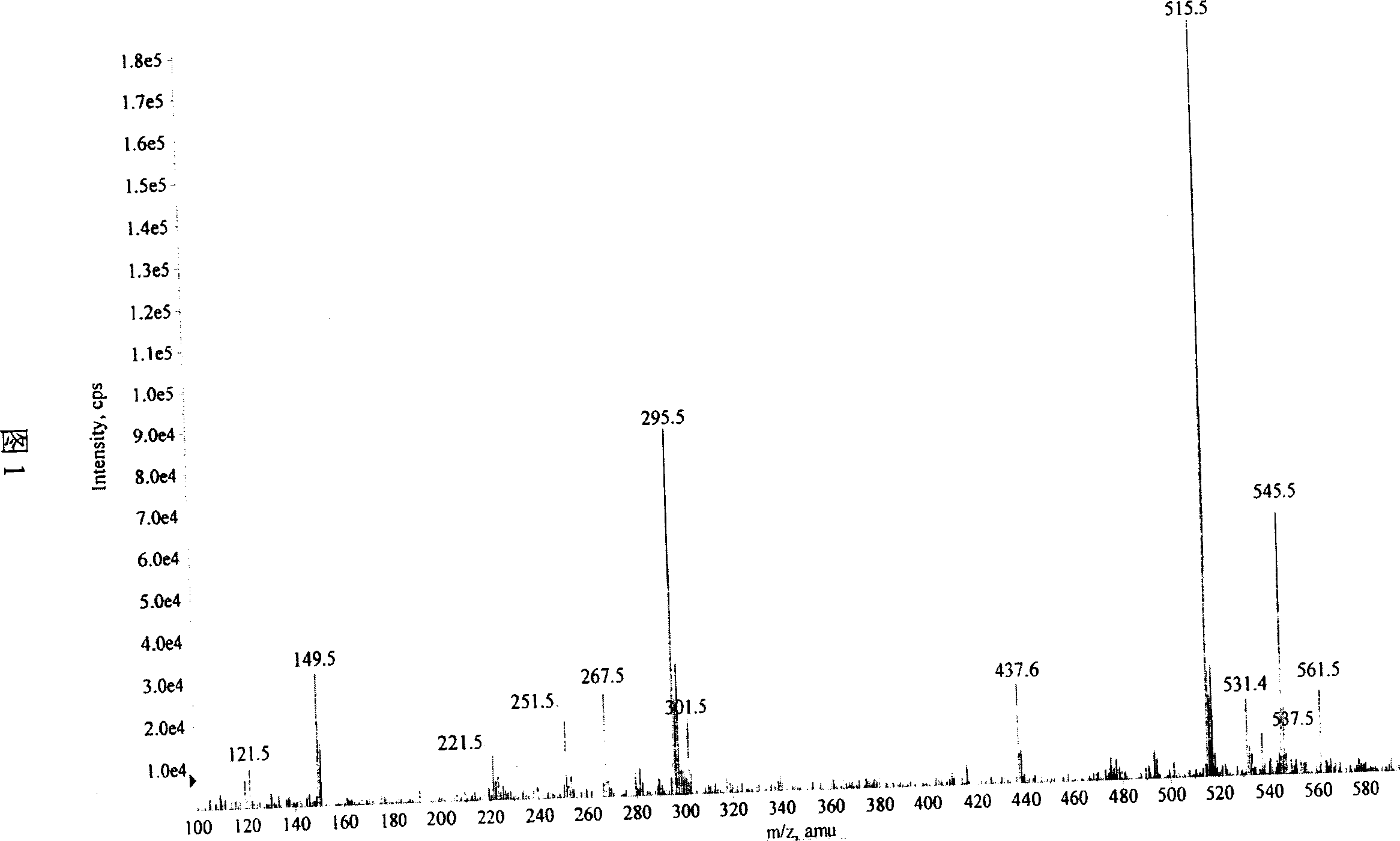
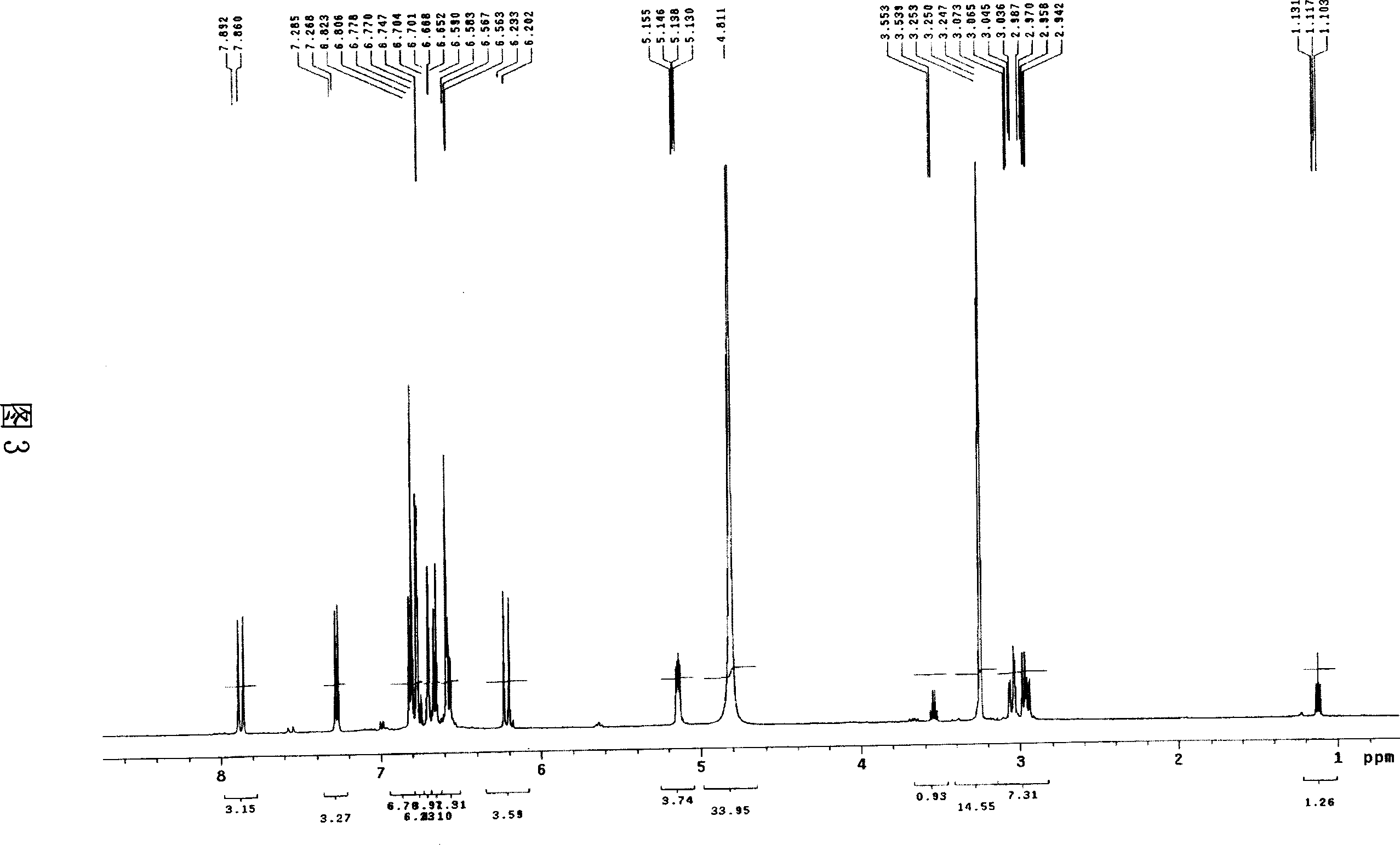
[0028] Salvianolic acid N: yellow amorphous powder, C 26 h 20 o 10 , with 1% FeCl 3 The reagent reaction is brown, ESI-MS (Figure 1): 515 (M+Na) + 、493(M+H) + 、295(M-197-Na) + . 1 HNMR (CD 3 OD)δ (Figure 3): 7.88 (1H, d, J = 16.0Hz, H-7) and 6.22 (1H, d, J = 16.0Hz, H-8) are a set of trans double bond proton signals, which The spectrum of gHMBC (Fig. 6) suggests that this group of trans double bond protons is conjugated to the carbonyl, 7.27 (1H, d, J=8.5Hz, H-7″) and 6.81 (1H, d, J=8.5Hz, H- 8″) is a group of cis double bond proton signals, 3.05 (1H, dd, J=15.0 / 4.0Hz, Hα-7′), 2.96 (1H, dd, J=15.0 / 8.0Hz, H β -7') and 5.14 (1H, dd, J=8.0 / 4.0Hz, H-8'), suggesting that there is -CH(O)-CH in the compound 2 -Structural fragments, a set of ABX coupling systems that bind aromatic regions, 6.70 (1H, d, J = 1.5Hz, H-2'), 6.66 (1H, d, J = 8.0Hz, H-5') and 6.57 ( 1H, dd, J=1.5 / 8.0Hz, H-6'), indicating that there is a β-(3,4-dih...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com