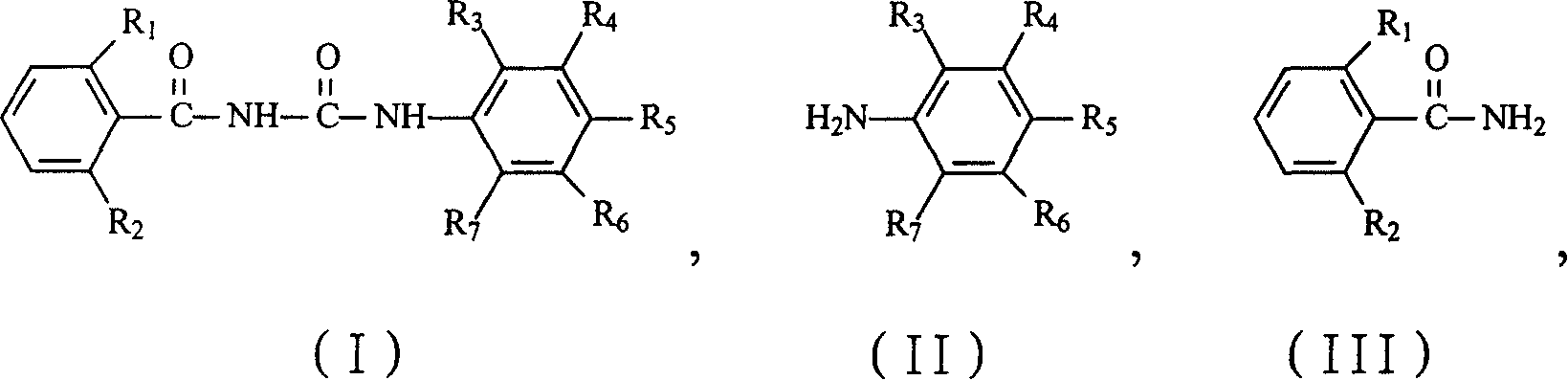
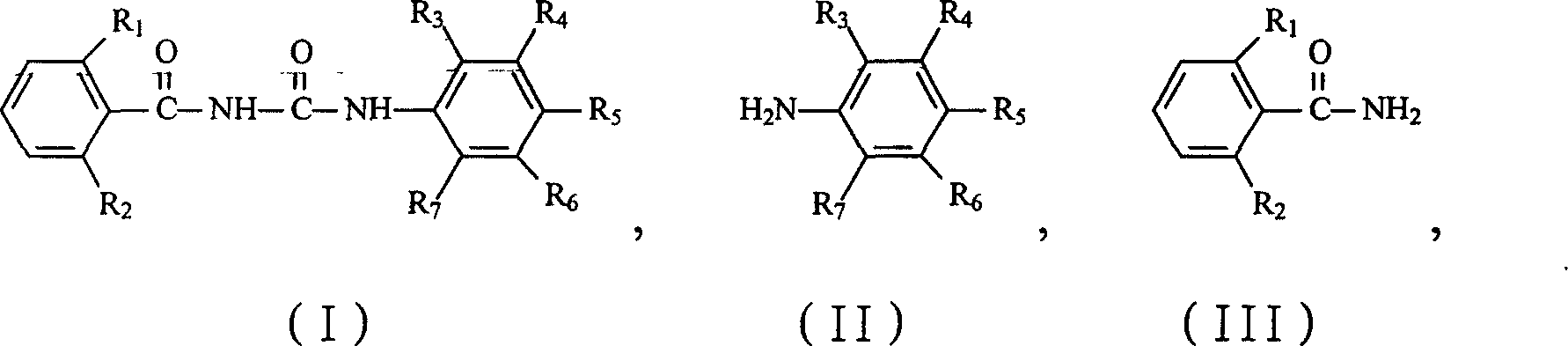
Preparation method of benzoyl area kind derivative
A technology for benzoyl urea and derivatives is applied in the field of preparation of benzoyl urea derivatives, which can solve the problems of large potential safety hazard, high production cost, equipment corrosion and the like, and achieves safe and reliable production, low production cost, less corrosive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 500mL three-necked flask, install a dropping funnel, a reflux condenser with a calcium chloride drying tube and a thermometer, and stir with a magnetic stirrer. Add 100mL xylene and 59.5g (0.2mol) bis(trichloromethyl)carbonate (BTC) into a three-necked flask, cool to below 0°C in an ice bath, stir, add 99.0g (0.5mol) 3,5-bis The mixture of chloro-2,4-difluoroaniline and 50 mL of xylene was slowly dropped, and the dropping temperature was controlled at 5-10°C. After the dropping, the temperature was raised to 90°C and reacted for 3 hours. Naturally cool, slowly add a mixture of 78.6g (0.5mol) 2,6-difluorobenzamide and 50mL xylene, add dropwise and raise the temperature to 100℃ to react for 7h, stop the reaction, cool naturally, filter, and dry in vacuo to obtain 181.2g of white needle-like solid, namely the product 1-(3,5-dichloro-2,4-difluorophenyl)-3-(2,6-difluorobenzoyl)urea, the yield is 93.5%, Analyzed by high performance liquid chromatography, the purity was 98.3%...
Embodiment 2
[0022] The amount ratio of the feed materials is 3,5-dichloro-2,4-difluoroaniline: bis(trichloromethyl) carbonate is 1:0.5, and the other operations are the same as in Example 1. The yield was 94.2%, and the purity was 98.6% by high performance liquid chromatography.
Embodiment 3
[0024] The quantity ratio of the feed materials is 3,5-dichloro-2,4-difluoroaniline: bis(trichloromethyl) carbonate is 1:0.6, and the other operations are the same as in Example 1. The yield was 94.7%, and the purity was 98.7% by high performance liquid chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com