Herbicidal pyrimidines
A C1-C4, C2-C4 technology, applied in the field of preventing and controlling unwanted plants, can solve the problems of production reduction and consumer cost increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0079] The embodiments of the present invention include:
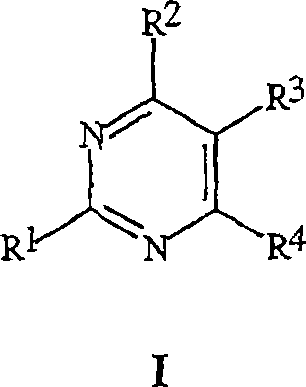
[0080] Embodiment 1. The compound of formula I, wherein j is zero.
[0081] Embodiment 2. The compound of formula I, wherein k is zero.
[0082] Embodiment 3. The compound of formula I, wherein R 15 It's H.
[0083] Embodiment 4. The compound of Embodiment 3, wherein R 16 It's H.
[0084] Embodiment 5. The compound of formula I, wherein
[0085] R is CO 2 R 12 , CH 2 OR 13 , CH(OR 46 )(OR 47 ), CHO, C(=NOR 14 )H, C(=NNR 48 R 49 )H, C(=O)N(R 18 )R 19 , C(=S)OR 50 , C(=O)SR 51 , C(=S)SR 52 Or C(=NR 53 )YR 54 ;
[0086] R 12 Is H, -CH_C(O)O(CH 2 ) m _, -N=C(R 55 )R 56 ; Or selected from C 1 -C 14 Alkyl, C 3 -C 12 Cycloalkyl, C 4 -C 12 Alkylcycloalkyl, C 4 -C 12 Cycloalkylalkyl, C 2 -C 14 Alkenyl, C 2 -C 14 Alkynyl and phenyl groups, each group is optionally substituted by 1-3 R 27 Replaced by; or
[0087] R 12 Is the carboxylate functional group CO that is bonded to each of the two pyrimidine ring systems in formula I 2 R 12 T...
Embodiment approach 1-98
[0210] Illustrate the combination of embodiments 1-98:
Embodiment approach A
[0211] Embodiment A. The compound of Formula I, wherein
[0212] R 2 Is CO 2 R 12 , CH 2 OR 13 , CH(OR 46 )(OR 47 ), CHO, C(=NOR 14 )H, C(=NNR 48 R 49 )H, (O) i C(R 15 )(R 16 )CO 2 R 17 , C(=O)N(R 18 )R 19 , C(=S)OR 50 , C(=O)SR 51 , C(=S)SR 52 Or C(=NR 53 )YR 54 ;
[0213] R 12 Is H, -CH_C(O)O(CH 2 ) m _, -N=C(R 55 )R 56 ; Or selected from C 1 -C 14 Alkyl, C 3 -C 12 Cycloalkyl, C 4 -C 12 Alkylcycloalkyl, C 4 -C 12 Cycloalkylalkyl, C 2 -C 14 Alkenyl, C 2 -C14 Alkynyl and phenyl groups, each group is optionally substituted by 1-3 R 27 Replace; or
[0214] R 12 Is the carboxylate functional group CO which is bonded to each of the two pyrimidine ring systems in formula I 2 R 12 The divalent group is selected from -CH 2 -, -(CH 2 ) 2 -, -(CH 2 ) 3 -And-CH(CH 3 )CH 2 -;
[0215] R 13 Is H, optionally by 1-3 R 28 Replaced C 1 -C 10 Alkyl, or benzyl;
[0216] R 14 Is H, C 1 -C 4 Alkyl, C 1 -C 4 Haloalkyl or benzyl;
[0217] R 17 Is optionally 1-3 R 29 Replaced C 1 -C 10 Alkyl, or benzyl;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com