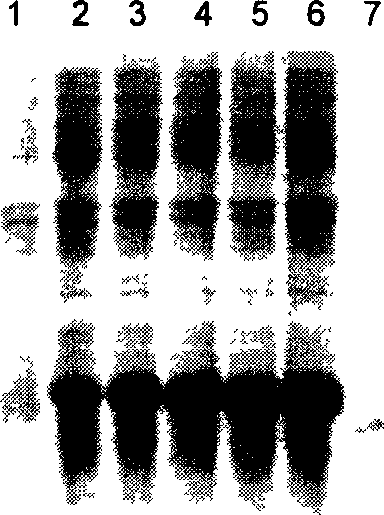Human pancreas hyperglycemiacin relative peptide-2 analogue
A technology for glucagon and related peptides, which is applied in the direction of glucagon, hormone peptides, animal/human proteins, etc., and can solve the problem of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
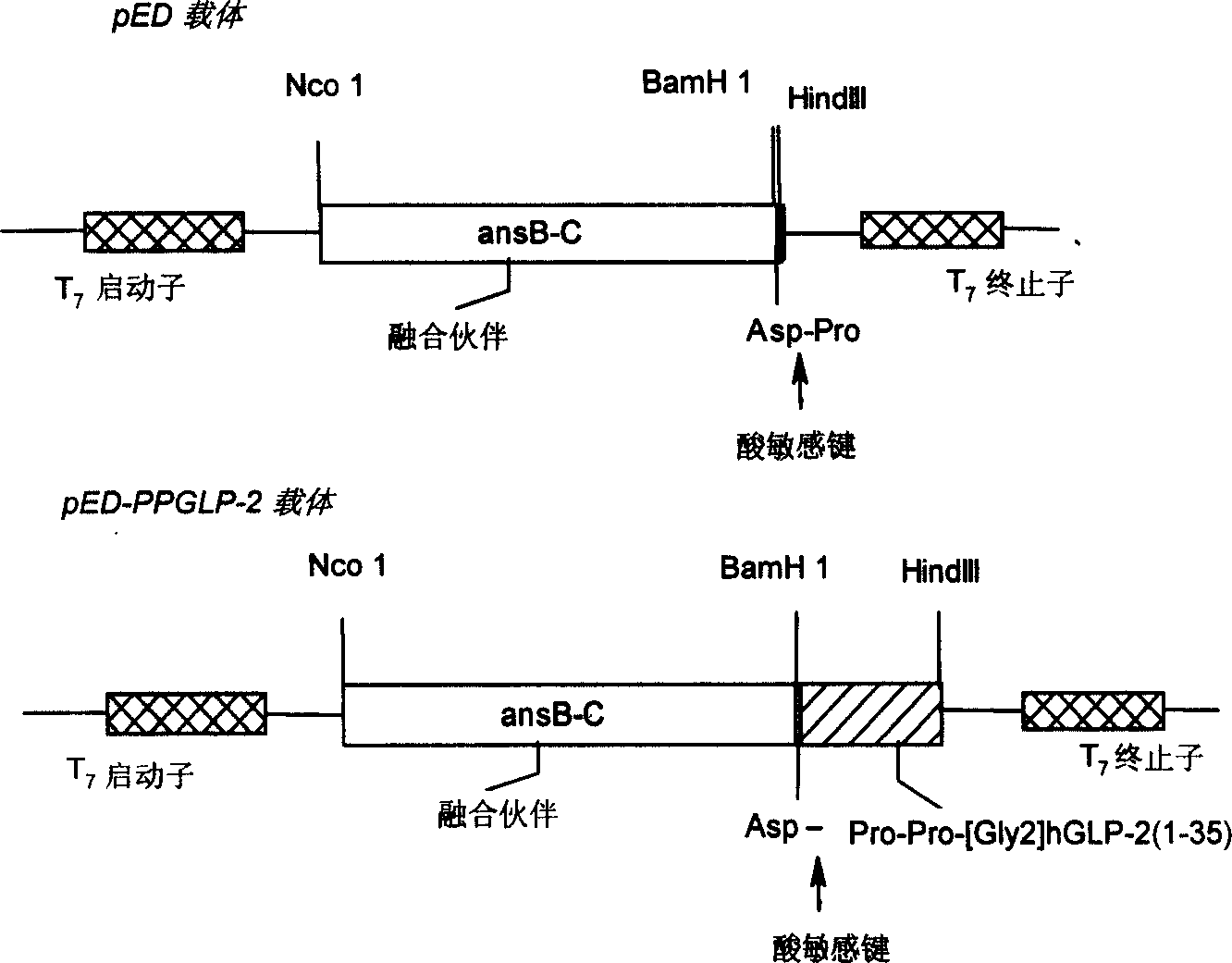
[0043] Embodiment 1Pro-Pro-h[Gly 2 ] Design, synthesis and cloning of GLP-2(1-35) polypeptide gene
[0044] According to codon and design requirements of E. coli, the alanine ( 2 The 35 amino acid sequence of human glucagon-related peptide-2 in which the Ala) residue is replaced by Gly and 2 prolines are extended at the N-terminal is converted into a nucleotide sequence, and its structural framework is shown in FIG. 2 . A BamH I restriction site was added at the 5' end, and a stop codon TAA and a HindIII restriction site were added at the 3' end. After computer-assisted analysis, it was determined that the gene was 124 bp in length and divided into 53, 54, and 53 bases. For the three oligonucleotide fragments, at the same time, a downstream primer M4 was designed for the primary screening of positive clones by PCR.
[0045] Synthesize 4 sequences as follows:
[0046] M1 (5'GCGG G GAT CC G CCG CAC GGT GAC GGT TCT TTC TCT GAC GAA ATG AAC ACC ATC 3’, including BamH I restric...
Embodiment 2
[0053] Embodiment 2Pro-Pro-h[Gly 2 ]Expression of GLP-2(1-35) polypeptide gene in Escherichia coli
[0054] Recombinant expression strain PED-PPGLP-2 / BL21 was shaken overnight at 37°C in liquid LB medium, transferred to corn steep liquor fermentation medium with 2% inoculum, cultured at 37°C for 4 hours, and induced expression with a final concentration of 5 mmol / l lactose , Collect the fermented cells after 4h. Reserved samples were analyzed by 15% SDS-PAGE and thin-layer scanning. An obvious protein band appeared at the molecular weight of about 17700Da ( Figure 1B ), with the fusion protein AnsB-C-Pro-Pro-h[Gly 2 ] The GLP-2 theoretical calculation value is consistent. The fusion protein reached a stable maximum expression level 8 hours after induction, and Densitometric analysis showed that the fusion protein accounted for more than 40% of the total bacterial protein and formed inclusion bodies in the cell.
Embodiment 3
[0055] Embodiment 3 Fusion protein separation and purification
[0056] The engineered bacteria after induced expression were collected by centrifugation, and the bacteria were suspended in the wall-breaking solution (pH8.0 phosphate buffered saline, 0.02% lysozyme). After the bacteria were lysed, inclusion bodies were obtained, and the inclusion bodies were washed, dissolved in urea and The pure fusion protein AnsB-C-Pro-Pro-h [Gly 2 ]GLP-2( Figure 2A ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com