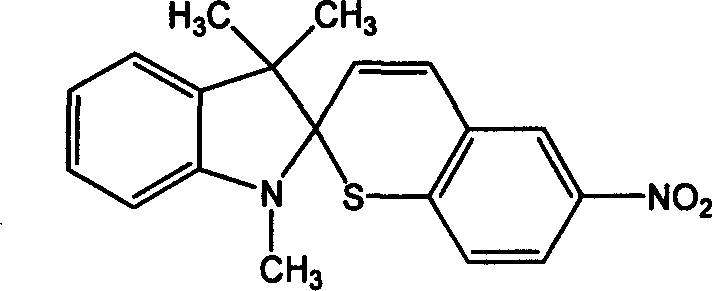
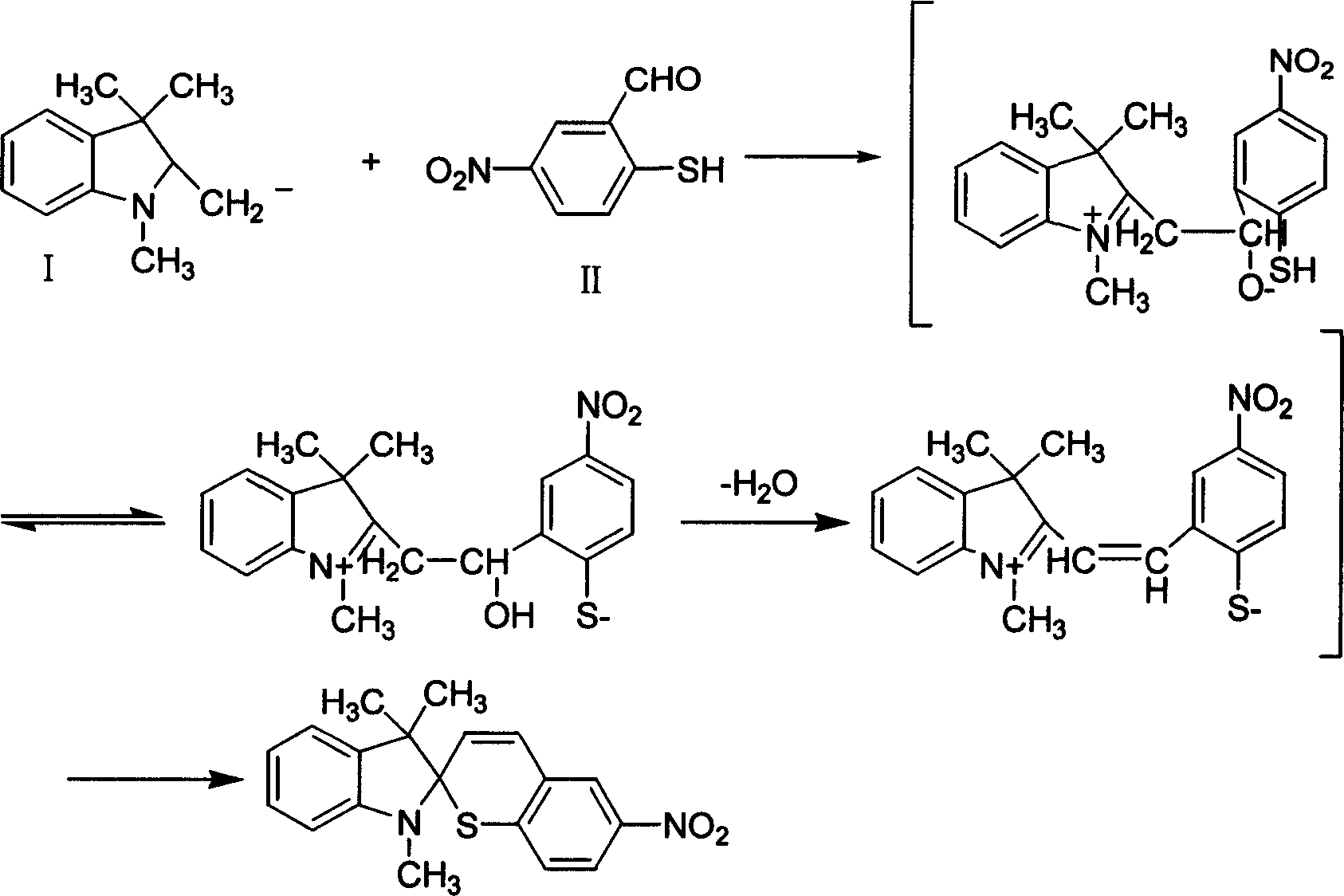
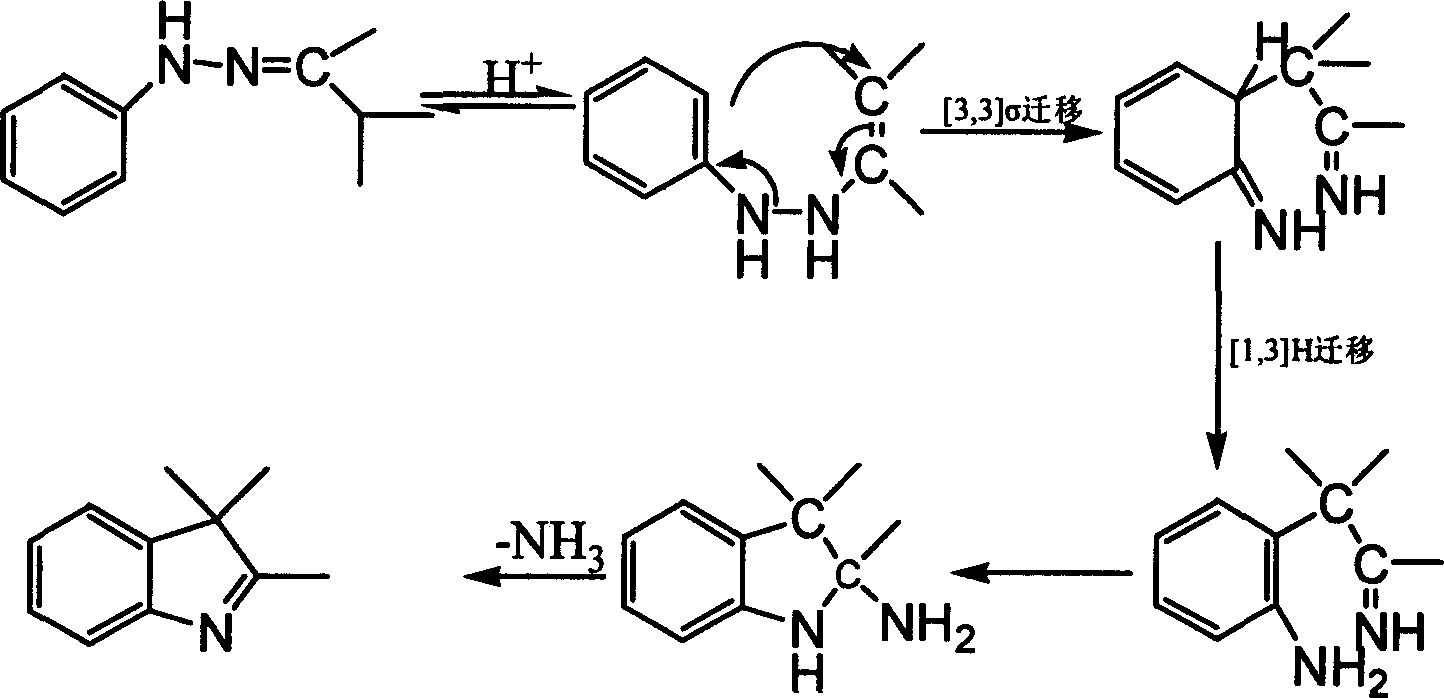
Preparation process of 6'-nitroindolyline benzspriothiane
A technology for benzospirothiopyran and nitroindoline, which is applied in chemical instruments and methods, organic chemistry, color-changing fluorescent materials, etc., can solve the problems of difficult industrial operation, high cost, harsh conditions for synthesizing spirothiopyran, and the like, Achieve the effect of low preparation cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] 2.1 Preparation of 5-nitrosalicylaldehyde
[0024] First prepare an ice-salt bath (ice:salt is about 3:1), and the temperature is about -20°C. Take o-chlorobenzaldehyde in a 250ml three-neck flask, cool it in an ice-salt bath, and slowly add concentrated sulfuric acid dropwise therein under vigorous stirring, and the reaction solution turns light yellow at this time. Continue to add mixed acid (10ml fuming nitric acid+14ml concentrated sulfuric acid) to it, and the reaction solution slowly turns dark red. Keep the temperature for 3h. Slowly pour the reaction solution into a beaker filled with ice cubes while stirring, a large amount of yellow precipitates are formed. Suction filtration and drying gave a light yellow solid. After recrystallization from absolute ethanol, 5-nitrosalicylaldehyde was obtained which was close to white.
[0025] 2.2 Preparation of 25-nitrothiosalicylaldehyde
[0026] Weigh Na 2 S·9H 2 O was added to a 100ml three-necked flask, and disti...
Embodiment
[0033] Add 9.2ml of 3-methyl-2-butanone to 9.2g of freshly distilled phenylhydrazine, and the reaction is exothermic. The oil bath was heated to reflux at 80°C for 4 hours. After the reaction, separate the layers, discard the water layer, and wash the oil layer with anhydrous MgSO 4 After drying, 13.5 g of crude hydrazone was obtained. Add 30ml of glacial acetic acid to the hydrazone obtained above, react in a 90°C water bath for 3 hours, raise the temperature to 150°C, and evaporate most of the solvent, about 16ml. Cool to room temperature, wash with saturated NaCO 3 The solution neutralizes the reaction solution. The aqueous and organic phases are separated. The aqueous phase was extracted with diethyl ether, and the extract was combined with the organic phase and washed with anhydrous MgSO 4 dry. Distill at normal pressure to collect the solvent, then distill under reduced pressure to collect the fraction at 85-90°C / 0.25Kpa to obtain 6.9g of light yellow oily liquid, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com