Mesothelin vaccines and model systems
A mesothelin and vaccine technology, applied in the direction of antibody medical components, carrier-bound antigen/hapten components, peptide/protein components, etc., can solve the problem of inability to generate T cell lines, cloning, and clinical response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] In order to identify genes that can be potential immune targets for most pancreatic cancer patients, we only focused on those unmutated genes that were overexpressed in most pancreatic cancer patients and overexpressed in vaccine cell lines. The first gene on this list is mesothelin (20, 21). For comparison and confirmation, we also studied prostate stem cell antigen (PSCA). SAGE data confirm that pancreatic cancer will express PSCA at levels similar to mesothelin (22).
[0097] We use a combination of two publicly available computer algorithms (23-25) to predict peptide nonamers that can bind to three common human leukocyte antigen (HLA) class I molecules. All 14 patients treated with the allogeneic GM-CSF vaccine expressed at least one of these HLA class I molecules (Table 2). The prediction algorithm "BIMAS" ranks possible HLA binding epitopes based on the predicted half-life dissociation of the peptide / HLA complex (23). The "SYFPEITHI" algorithm ranks peptides based on ...
Embodiment 2
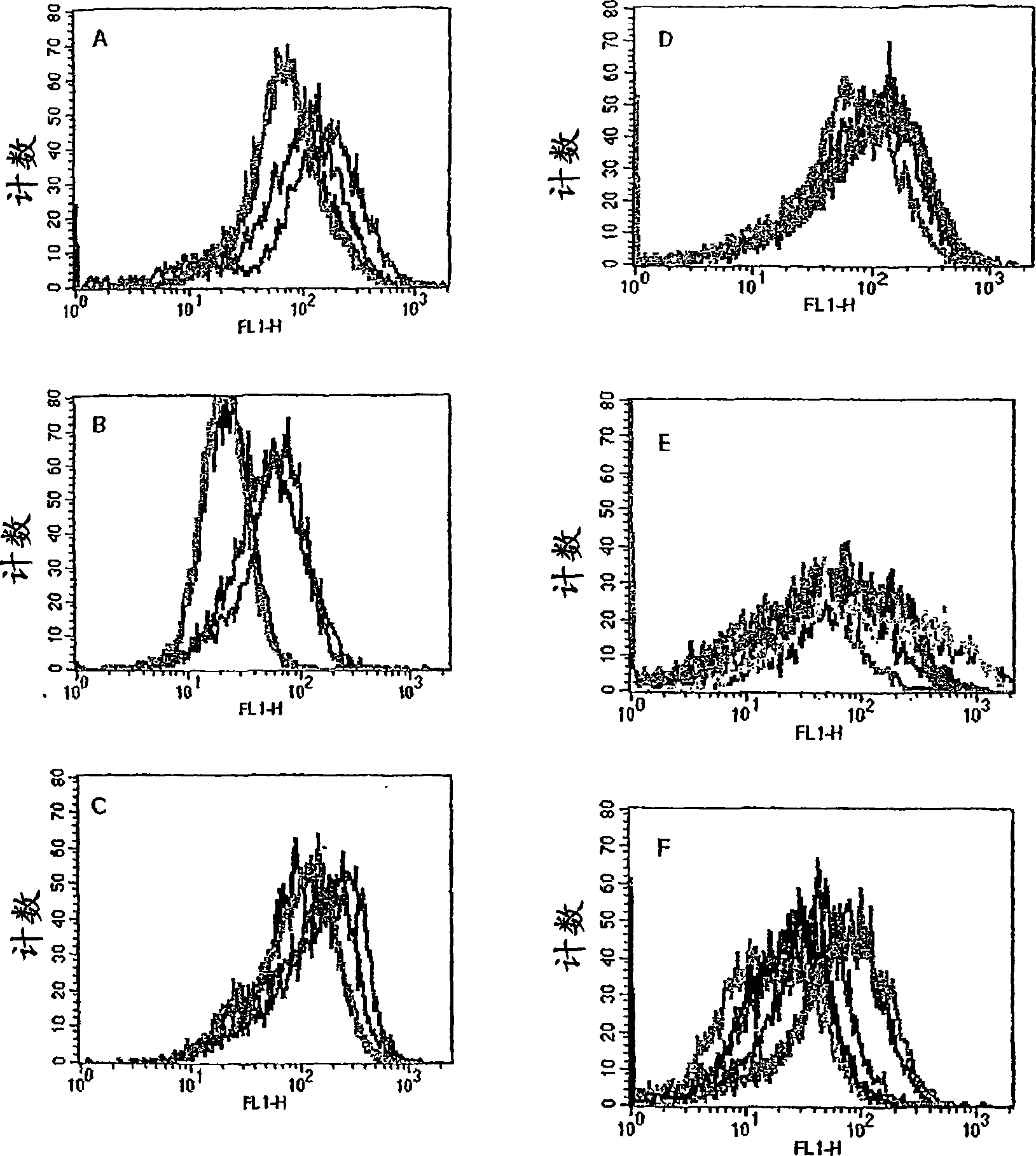
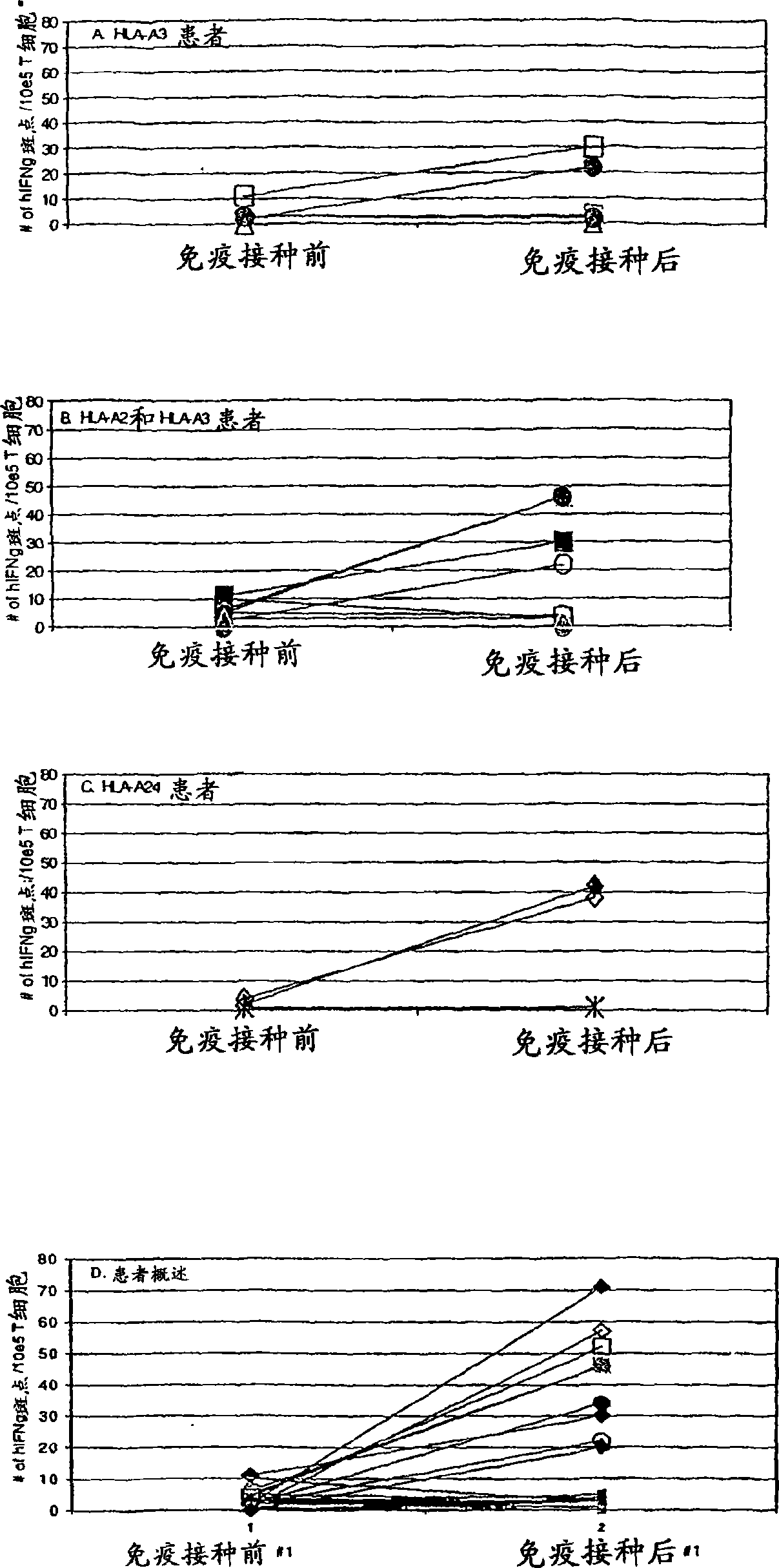
[0110] To determine whether mesothelin and PSCA are affected by CD8 + T cell recognition, we use the patient’s CD8-rich + The PBL of T cells screens antigen-pulsed T2 cells, and these patients receive an allogeneic pancreatic cancer vaccine that secretes GM-CSF. We previously reported that in 3 of 8 patients who received up to two vaccines, delayed-type hypersensitivity (DTH) reactions in vivo after vaccination were associated with autologous tumors. These "DTH responders" (they all have poor prognostic indicators at the first surgical resection) (27) are only patients who have not had pancreatic cancer clinically for more than 4 years after diagnosis ((27), Table 2). The PBL obtained before vaccination and 28 days after the first vaccination was analyzed initially. T2-A3 cells pulsed with two A3 binding epitopes are enriched with peripheral blood isolated from patient 10 (non-DTH responder, relapsed 9 months after diagnosis) and patient 13 (DTH responder, still disease-free) CD8 ...
Embodiment 3
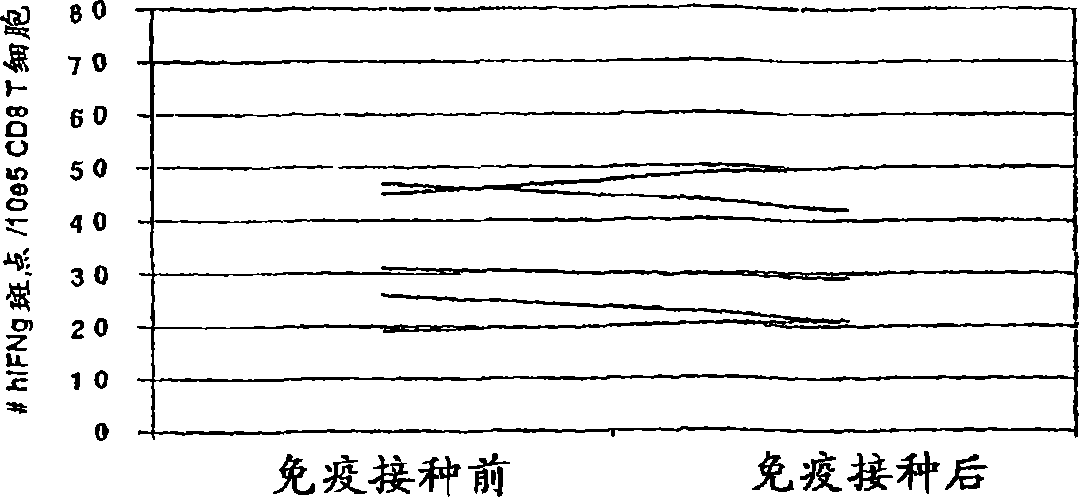
[0116] The above data clearly demonstrates the in vivo DTH response to autologous tumors and the long-term disease-free survival and mesothelin-specific CD8 + Correlation between post-vaccination induction of T cell responses. However, this correlation may be systemic immunosuppression (for patients who cannot prove DTH response to autologous tumors and disease progression after vaccination), rather than vaccine-specific induction of mesothelin in patients who remain disease-free DTH response T cell response. To prove CD8 specific for mesothelin + The induction of T cells after vaccination is tumor antigen-specific, and we evaluated the T cell response of each HLA-A2-positive patient to HLA-A2-binding influenza matrix peptide M1 (28). We chose the influenza M1 peptide because most of the patients in the vaccine study received an influenza vaccine some time before participating. Such as image 3 As shown, all HLA-A2-positive patients demonstrated similar pre-vaccination and post-va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com