Mono-cloning antibody of anti-human cancer protein iASPP and use thereof
A monoclonal antibody, oncoprotein technology, applied in anti-animal/human immunoglobulin, recombinant DNA technology, introduction of foreign genetic material using vectors, etc., can solve the problem of lack of antibodies, and achieve the effect of promoting the pathogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Cloning of embodiment 1 human iASPP gene and construction of eukaryotic and prokaryotic expression vectors
[0028] 1.1 Cloning of human iASPP gene
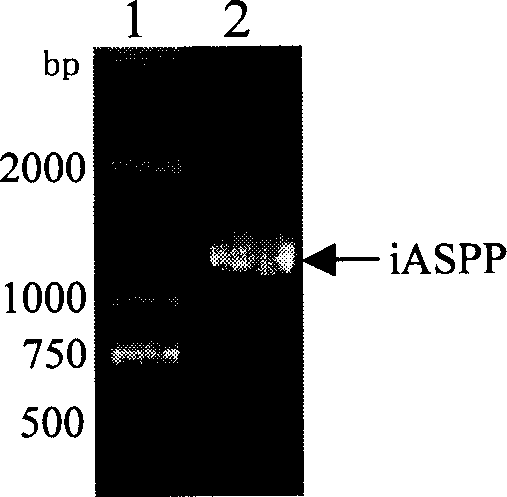
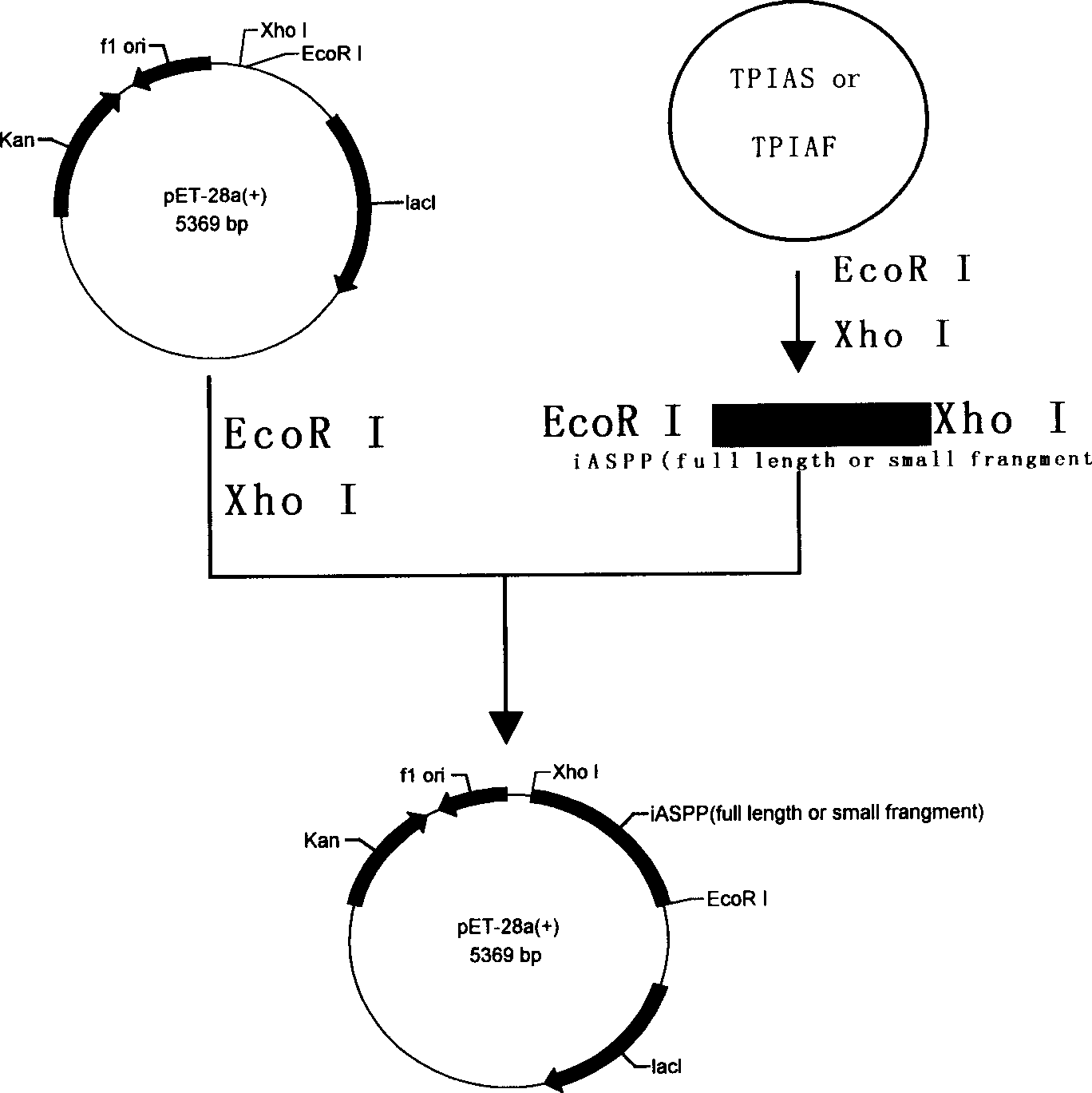
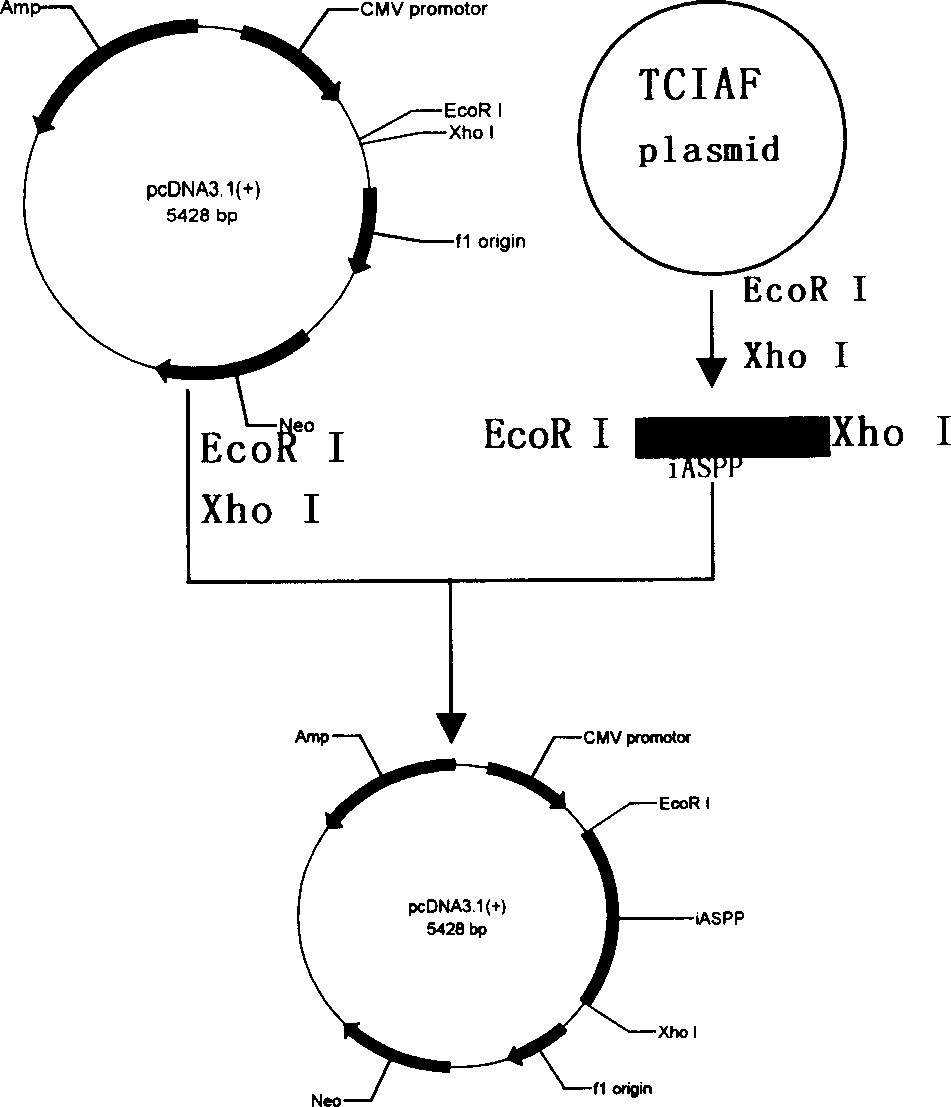
[0029] Collect U-937 cells in the logarithmic growth phase by centrifugation (1-5)×10 6 , TRIzol extracted total RNA, and measured A with a spectrophotometer 260 / A 280 , Calculate the RNA content, and confirm the integrity of the RNA by 12g / L agarose gel electrophoresis. 20μl reverse transcription reaction system containing 2μg total RNA, 4μl RT buffer, 50pmol / L oligo(dT) 16 , 1 μmol / L dNTPs, 0.1 μmol / L DTT, 15 U RNase inhibitor (Takara), 200 U MLV (Invitrogen), in 65 ° C water bath for 5 min, ice bath for 5 min, 37 ° C water bath for 60 min, 70 ° C for 15 min to stop the reaction, the obtained cDNA Store at -20°C for later use. According to the iASPP CDS sequence (NM_006663), use the gene runner software to design primers, add EcoR I to the 5' end of the antisense strand and add Xho I restriction site to the 5' end ...
Embodiment 2
[0034] Example 2 Expression and purification of screening antigens
[0035] 2.1 The PIAF and PIAS plasmids obtained in Example 1.2 were respectively transformed into Rosetta (DE3) prokaryotic expression strains, and positive clones were screened on kanamycin and chloramphenicol double-resistant LB medium agar plates. Pick a single clone and inoculate it in LB medium containing kanamycin (50mg / L) and chloramphenicol (34mg / L), and culture it with shaking at 37°C until OD 600 Reach 0.6-0.8. Inoculate in LB medium containing the same concentration of antibiotics at a ratio of 1:100, culture with shaking at 37°C until OD 600 After reaching 0.6-0.8, add IPTG to a final concentration of 1 mmol / L to induce expression. After 4 hours, the cells were collected by centrifugation at 4°C. Resuspend in an appropriate amount of 1×SDS loading buffer, place in a boiling water bath for 3-5 minutes, and take 50 μl for SDS-PAGE electrophoresis analysis. The molecular weights of the target frag...
Embodiment 3
[0040] The preparation of embodiment 3 monoclonal antibody
[0041] 3.1 Animal immunity
[0042] The CIAF plasmid obtained in Example 1.3 was extracted using a large extraction plasmid kit, and the plasmid was diluted with PBS to a concentration of 100 μg / 100 μl and injected into the spleen of mice, 100 μl per mouse.
[0043] 3.2 Preparation of feeder cells
[0044] The feeder cells used in the present invention are mouse peritoneal macrophages. The feeder cells were prepared the day before fusion, and the preparation process was as follows:
[0045] Use the same strain of Balb / c mice as the immunized mice, aged 6-8 weeks →
[0046] Kill the mice by cervical dislocation, soak them in 75% alcohol, and disinfect them for 3-5 minutes →
[0047] Mount the mouse on a dissecting board, cut the skin with sterile scissors, and expose the peritoneum→
[0048] Inject 6-8ml of culture solution into the abdominal cavity with a sterile syringe→
[0049] Repeatedly flush the peritonea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com