Milk fractions and milk preparations for treating and/or preventing COX-2 mediated diseases
A product, mediated technology, applied in the field of dairy ingredients and dairy products for the treatment and/or prevention of COX-2 mediated diseases, capable of solving problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
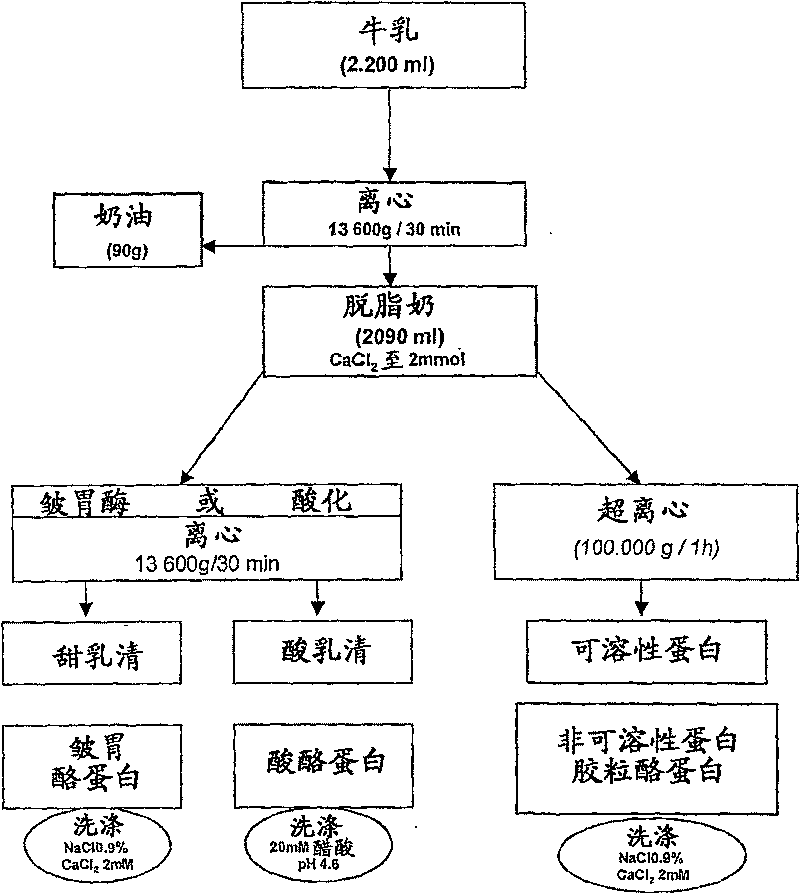
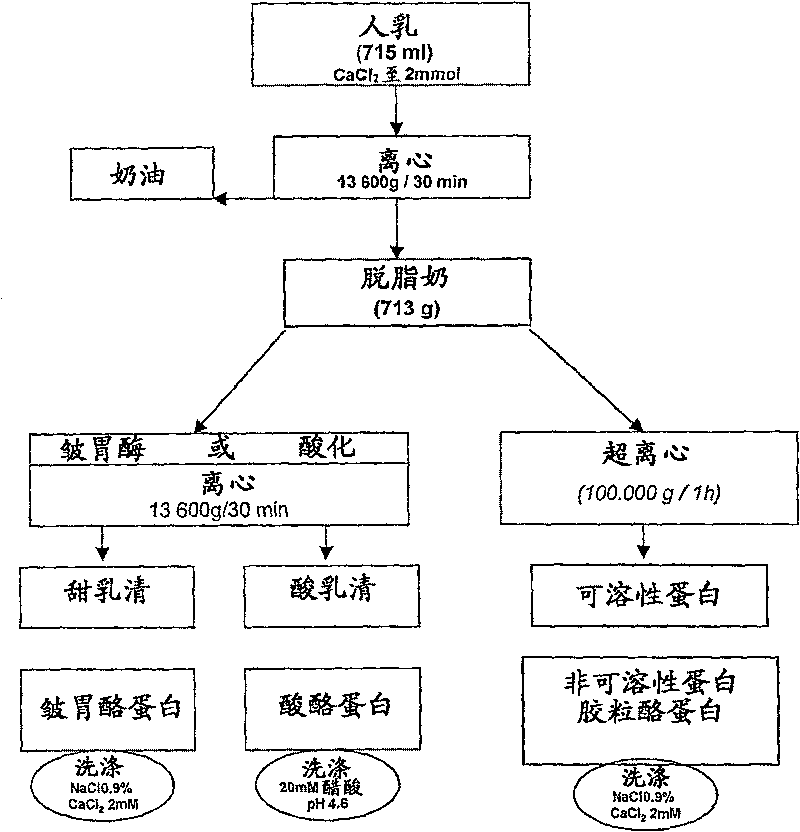
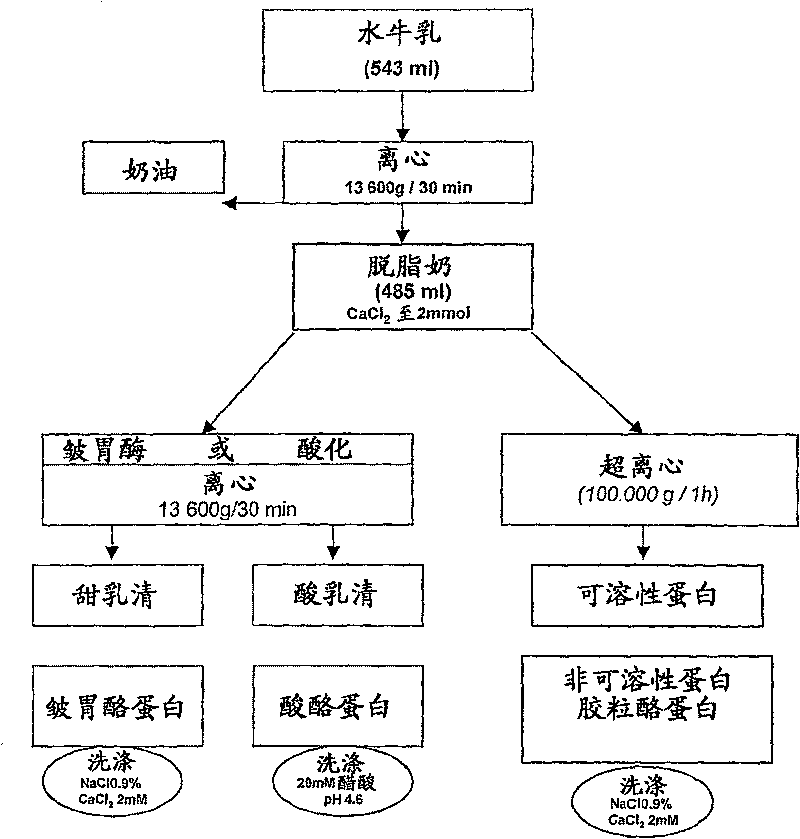
[0068] Milk sample preparation
[0069] Standard procedures for the preparation of dairy ingredients were carried out as described in C. Alais, Science du Lait, Principles Techniques Laitieres. SEPAIC, Paris, 4th Edition, 1984, pp. 29-35 and 159-178, unless expressly stated.
[0070] The described laboratory-scale fractionation procedure was developed from traditional industrial dairy processing methods. Surprisingly, the selection and separation efficiency can be significantly increased by carrying out the method steps described below, in particular performing high-acceleration centrifugation and particularly modified washing steps.
[0071] 1.1 milk
[0072] Milk was purchased from a local market ("Toni lait", Switzerland).
[0073] In the first step, cream is extracted from whole milk by centrifugation at accelerations up to 13 600 g (9 000 rpm for 30 min) using a fixed-angle rotor, Sorval GS3. Starting from 2.200ml of whole milk, 90g of cream is recovered from the t...
Embodiment 2
[0119] Subfractionation of Soluble Whey Proteins in Bovine Milk
[0120] Melt 15ml of the soluble whey protein obtained as described in Example 1 in a 37°C water bath for 20 minutes, shake and mix, and centrifuge in a 5415 Eppendorf centrifuge at 13.000rpm for 1 minute. After filtering with a 0.45 μm Millipore filter (306 / GSWP04700.GS), 10 ml of the product was injected into an HR16×50 column filled with 100 ml of Source 15RPC TN 17-0727-02 (polystyrene-divinylbenzene). The column was connected to an FPLC system controlled by a UNICORN workstation (AmershamPharmacia Biotech).
[0121] Chromatography conditions are:
[0122] Buffer A: 0.1% TFA in water (2000ml of miliQ water filtered by a 0.45μm Millipore system, plus 2ml of TFA (Sigma 91699, 100ml));
[0123] Buffer B: 80% acetonitrile, 0.85% TFA (400ml miliQ water filtered by 0.45μm Millipore system, add 1.600ml acetonitrile, degas in ultrasonic bath for 15 minutes, and finally make up 1.7ml TFA).
[0124]The column was ...
Embodiment 3
[0135] COX-2 screening
[0136] 2.1 Materials and methods
[0137] HUV-EC-C cells (immortalized endothelial cell line derived from normal human umbilical cord vessels; ATCC CRL1730; M.Miralpeix, M.Camacho et al., "British Journal of Pharmacology" (Brit.J.Pharmacol.) 121( 1997), 171-180) were inoculated onto a 96-well plate (PRMI 1640+10% FCS), and treated with 100nM (nmol / l) phorbol 12-myristoyl 13-acetate at 37°C for 6 hours, To induce COX-2 isozyme. Subsequently, 50 μM (μmol / l) arachidic acid was added, and the cells were incubated at 37°C for 30 minutes in the presence of the test compound or mixture. ), radioactivity was determined by scintillation counter (Topcount, Packard).
[0138] The results obtained are expressed as percent of the control value and percent inhibition of the control value in the presence of the test compound. The control value is the concentration of HUV-EC-C cells stimulated with phorbol myristoyl acetate (PMA) (or alpha tumor necrosis facto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com