Inhibitors of P38 and methods of using the same
A compound, C3-C6 technology, applied in anti-inflammatory agents, drug combinations, non-central analgesics, etc., can solve problems such as not having oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
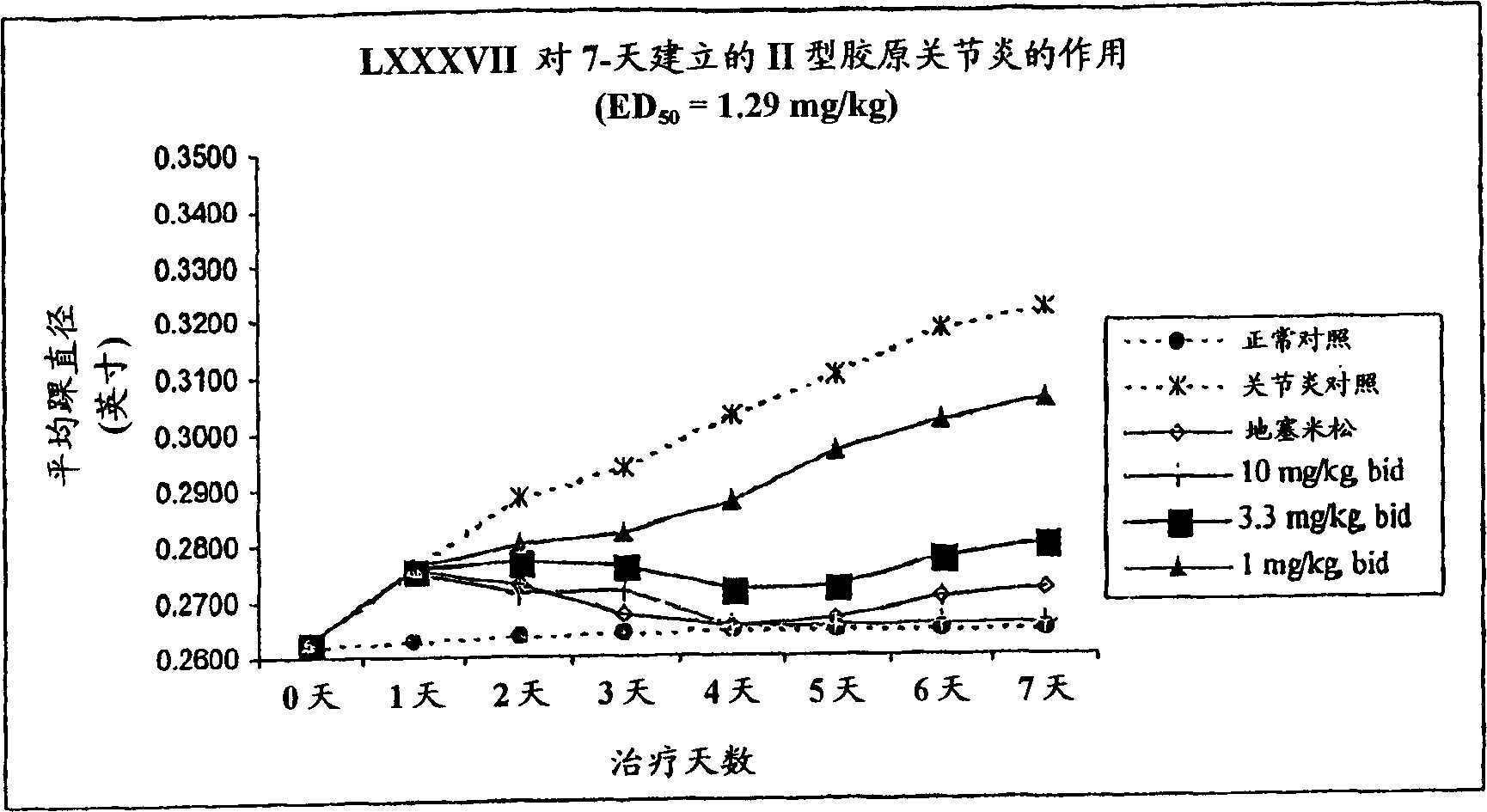
Examples
Embodiment 1
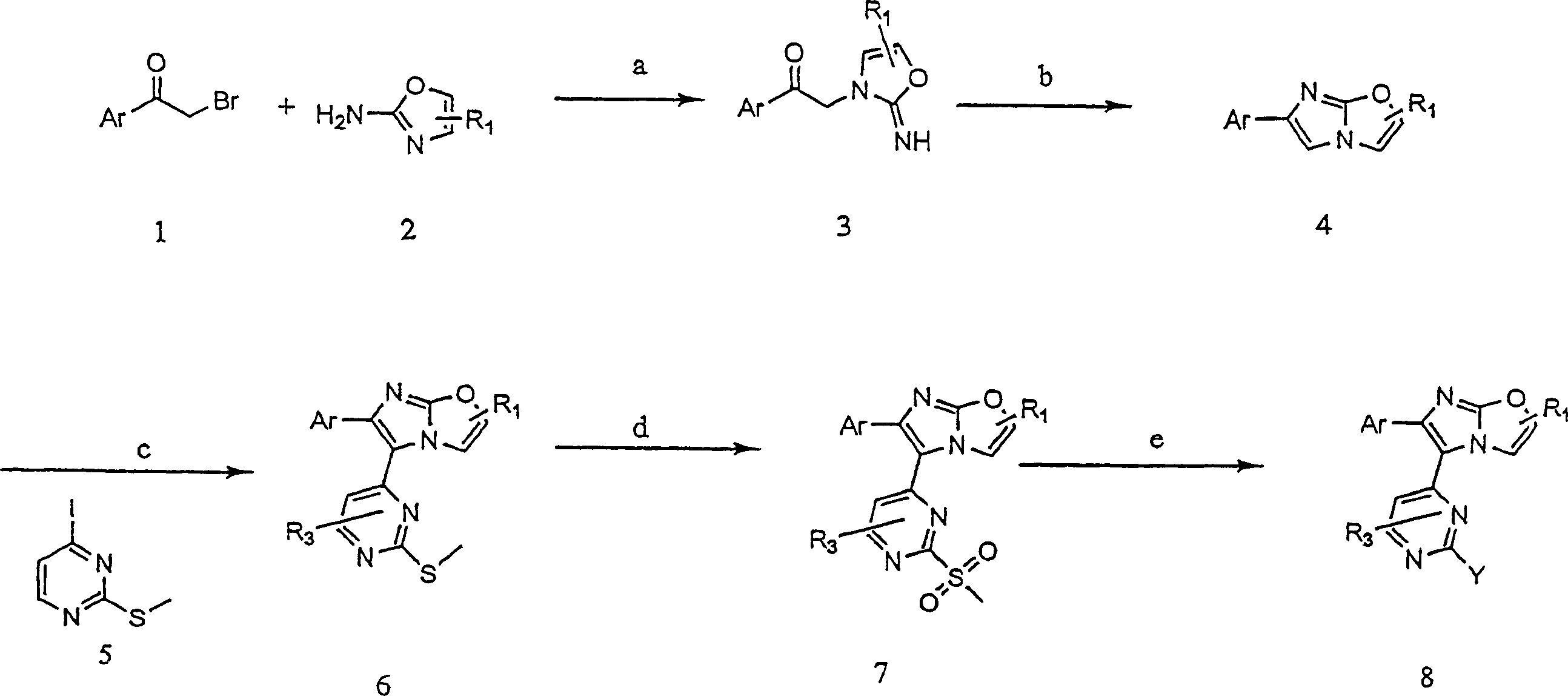
[0172] Example 1: 3-({4-[6-(4-fluorophenyl)imidazo[2,1-b][1,3]oxazol-5-yl]pyrimidin-2-yl}amino)- 2, Preparation of 2-dimethylpropan-1-ol
[0173] Compounds of the present invention represented by formula I (wherein X is O) are prepared according to the following representative methods.
[0174] Unless otherwise specified, the use of commercially available compounds is generally accepted.
[0175]
[0176] step 1
[0177] 2-amino-oxazole
[0178] To a solution of cyanamide (33 mL, 50% wt in water, 0.416 mol) in THF (100 mL) was added a solution of 2-hydroxyacetaldehyde (25 g, 0.416 mol) in water (40 mL), followed by dropwise addition of 2M sodium hydroxide (42 mL, 0.083 mol). Stirring was continued for a total of 24 hours. Then, the reaction mixture was concentrated in vacuo to remove most of the THF. The residual aqueous layer was extracted with ethyl acetate (4 x 200 mL). The extract was dried over sodium sulfate and the solvent was evaporated in vacuo. This gave p...
Embodiment 2
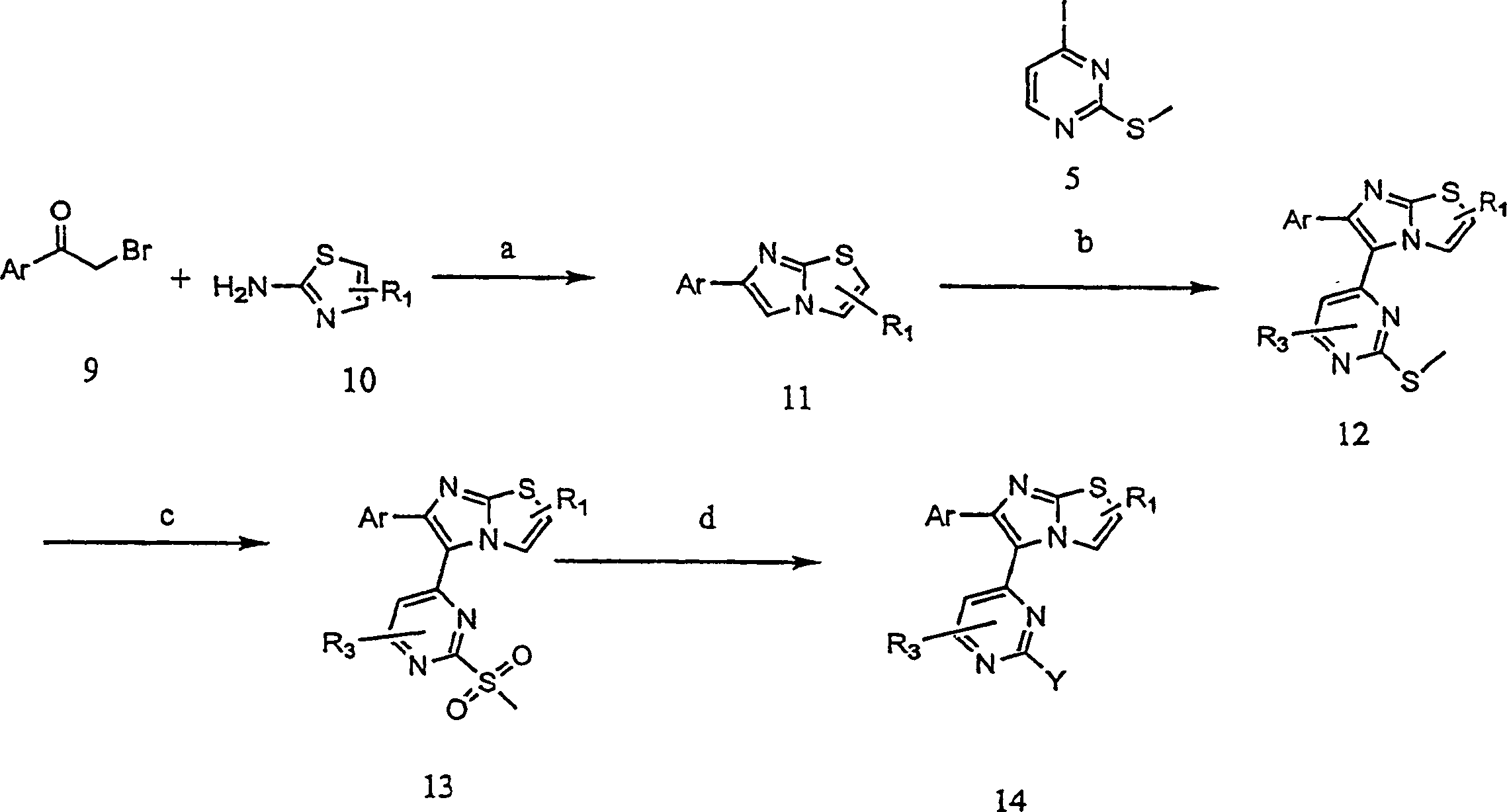
[0206] Example 2: 4-{4-[6-(4-fluorophenyl)-imidazo[2,1-b]thiazol-5-yl]-pyrimidin-2-ylamino}-piperidine-1-carboxy Preparation of tert-butyl acid
[0207] Exemplary compounds of the present invention represented by formula I (X=S) were prepared according to the following representative methods.
[0208] step 1:
[0209]
[0210] Add pure ethanol (600mL ). The reaction was refluxed for 16 hours with vigorous stirring. The reaction mixture was reduced to half its original volume in vacuo. The residual liquid was poured into ice and the solution became basic after addition of ammonium hydroxide solution (30%). The resulting fine solid was filtered and washed with water. The black-yellow solid thus obtained was dried in a vacuum electric furnace at 50° C. to obtain 6-(4-fluorophenyl)-imidazo[2,1-b]thiazole (J) (43.0 g, 86%).
[0211] ESMS[M+H] + = 219;
[0212] 1 H NMR (300MHz CDCl 3 )δ8-7.6 (m, 3H), 7.38 (bs, 1H), 7.08 (bs, 2H), 6.79 (bs, 1H); 13 C75MHz (NMR CDCl 3 ...
Embodiment 3
[0234] Example 3: N'-{4-[6-(4-fluorophenyl)imidazo[2,1-b][13]thiazol-5-yl]pyrimidin-2-yl}-N,N-two Preparation of methylethane-1,2-diamine
[0235]
[0236] To 6-(4-fluorophenyl)-5-[2(methylsulfonyl)pyrimidin-4-yl]imidazo[2,1-b][1,3]thiazole (L; see Example 2 above ) (200mg, 0.53mmol) in dry dimethylsulfoxide (4mL) was added N,N-dimethylethanolamine (273mg, 3.06mmol) and potassium carbonate (365mg, 2.64mmol). The reaction mixture was stirred at 100°C for 6 hours, diluted with water (5 mL), extracted with ethyl acetate (2 x 10 mL). The organic phase was washed with water, dried over anhydrous sodium sulfate, filtered and the solvent was evaporated in vacuo. The residue was purified by silica gel column chromatography to obtain the compound N'-{4-[6-(4-fluorophenyl)imidazo[2,1-b][1,3]thiazol-5-yl]pyrimidine-2 -yloxy}-N,N-Dimethylethylamine (N) (108 mg).
[0237] 1 H NMR (300MHz, DMSO) δ: 8.55(d, J=4.8Hz, 1H), 8.41(d, J=5.4Hz, 1H), 7.65(m, 2H), 7.52(d, J=4.5Hz, 1H ), 7.32...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com