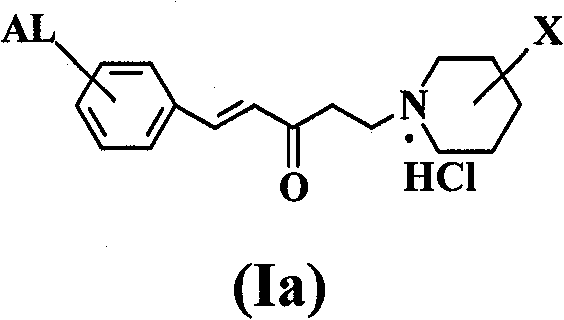
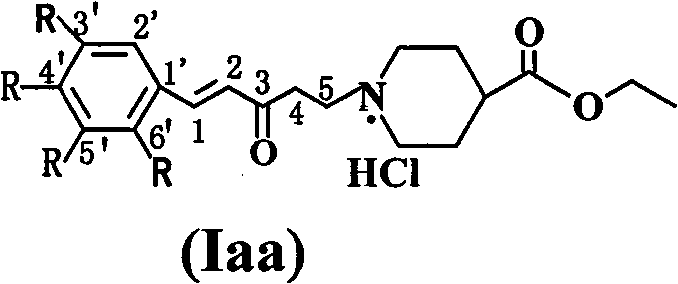
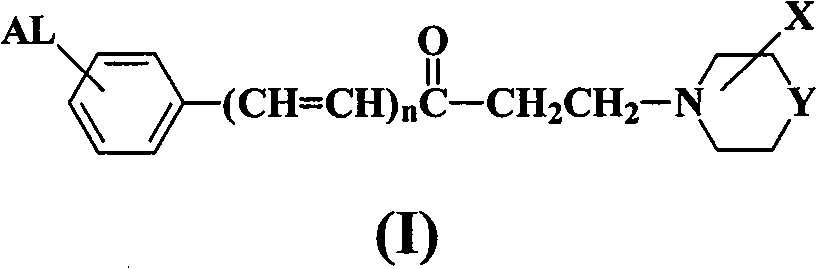Aromatic amine ketone compounds, its synthesis method, pharmaceutical composition containing same and uses
A compound and drug technology, applied in the field of arylamine ketone compounds, can solve serious side effects and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0115] Preparation Example 1. Preparation of 1-(4-chloro)phenyl-1-buten-3-one:
[0116] Add 8.43g of p-chlorobenzaldehyde, 60ml of acetone, and 6ml of ethanol into a 500ml beaker, add 240ml of water and 48ml of 10% sodium hydroxide aqueous solution under stirring, and gradually produce solids under rapid stirring, and the reaction is carried out at about 25°C. After checking the completion of the reaction (developing solvent: cyclohexane: ethyl acetate = 2:1), under cooling in an ice-water bath, add 6N hydrochloric acid dropwise to acidify to ph = 5-6, and then stir rapidly for 30 minutes. Let it stand at room temperature overnight, then suction filter the next day, wash the solid with a large amount of water, and dry it to obtain 10.5 g, mp.: 56-57°C, yield 96.9%. Preparation Example 2. Synthesis of 1-(4-hydroxy)phenyl-1-buten-3-one:
preparation example 2
[0117] Add 10g of 4-hydroxybenzaldehyde and 28ml of newly prepared 10% sodium hydroxide aqueous solution in a 500ml three-necked bottle, and under rapid stirring, most of the solids can be dissolved to obtain a light brown solution. After adding 20g of acetone, it becomes dark immediately Brown clear solution. Add 40ml of 10% aqueous sodium hydroxide solution, and check by TLC until the reaction is complete. Dilute with distilled water until all the solids are dissolved, add hydrochloric acid dropwise to acidify, a large amount of light yellow solids precipitate, stand for a while, then filter with suction, wash the solids with water, and after drying, wash the solids with 50% ethanol for 2 to 3 times to obtain pale yellow solids. Yellow solid, dried to obtain 11.06g, mp.: 98-101°C, yield 83.7%, recrystallized with 50% ethanol, decolorized with activated carbon, placed in refrigerator to precipitate crystals, filtered, dried to obtain light yellow crystals 9.3 g, mp: 104~105℃...
preparation example 3
[0118] Preparation Example 3. Preparation of 1-(4-methyl)phenyl-1-buten-3-one:
[0119] Dissolve 1.2g (10mmol) of 4-methylbenzaldehyde in 10ml of acetone, add 20ml of water under stirring, slowly drop into 4ml of 10% NaOH solution (internal temperature does not exceed 20°C), continue to react at room temperature for 14 hours, TLC shows that the reaction Complete, neutralized with 10% HCI to neutral, with CH 2 Cl 2 Extracted, washed with water, dried and concentrated to obtain 0.73g of light yellow viscous substance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| rate of recovery | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com