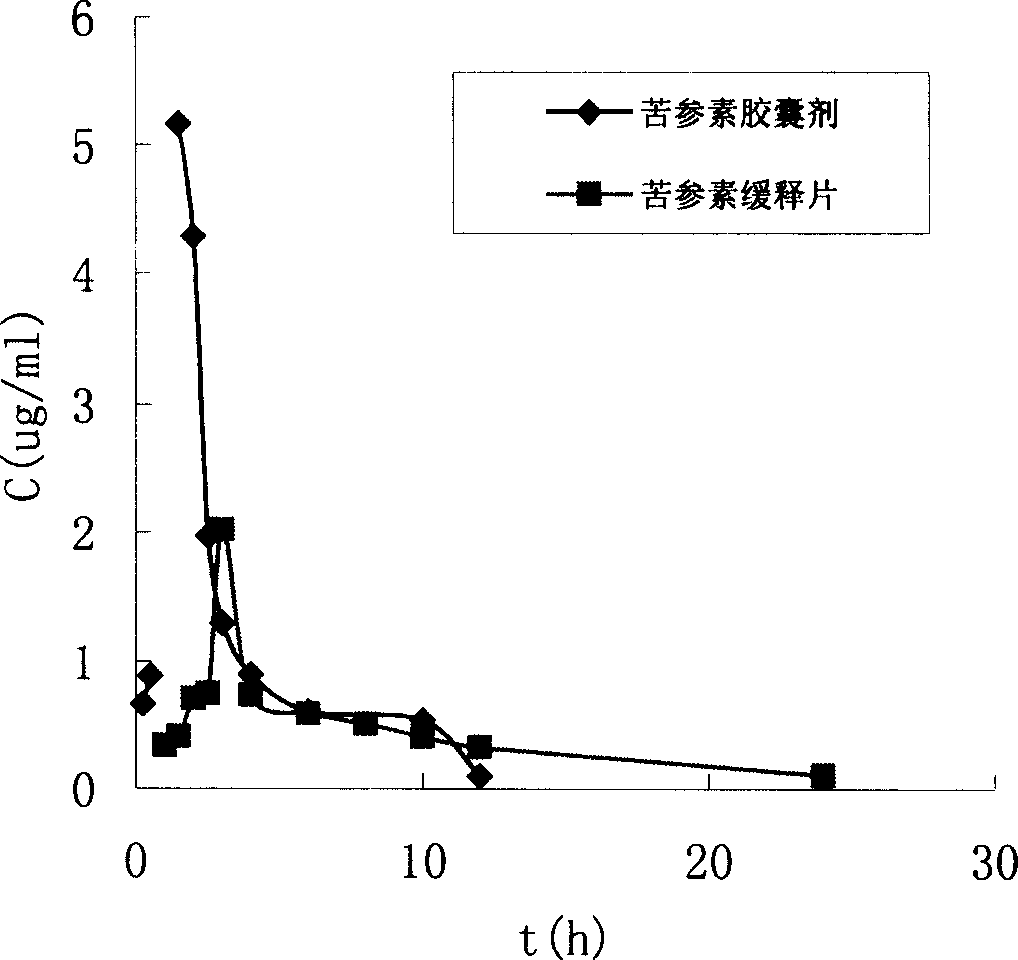
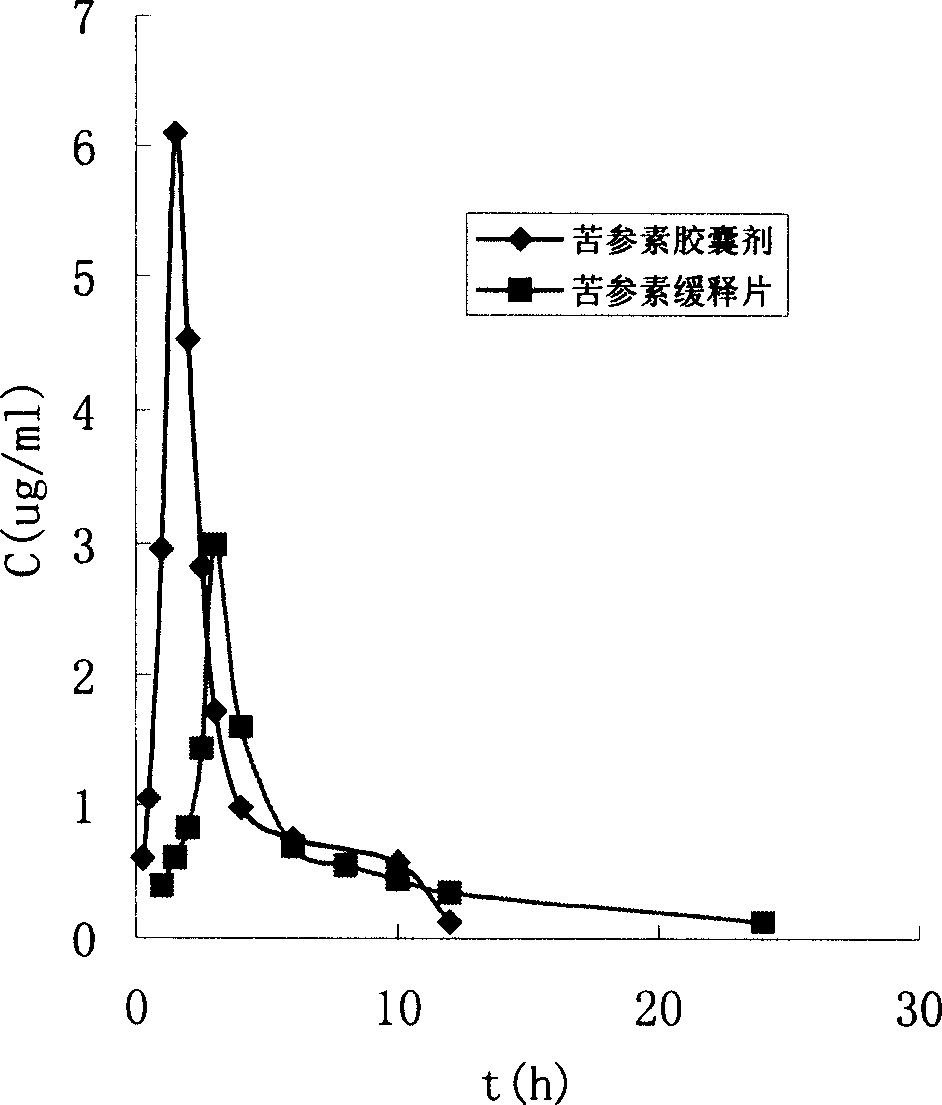
Kurarinone formulation and its preparation process
A technology of matrine and sustained-release preparations, applied in the field of matrine preparations and its preparation, which can solve the problems of poor compliance, long course of treatment for chronic hepatitis, difficulty for patients to adhere to long-term treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Take the raw materials according to the following weight ratio:
[0050] Matrine 400 parts
[0051] Hypromellose 300 parts
[0052] 90% ethanol solution 100 parts
[0053]Lactose 25 parts
[0054] Magnesium Stearate 8 parts
[0055] The production method is as follows:
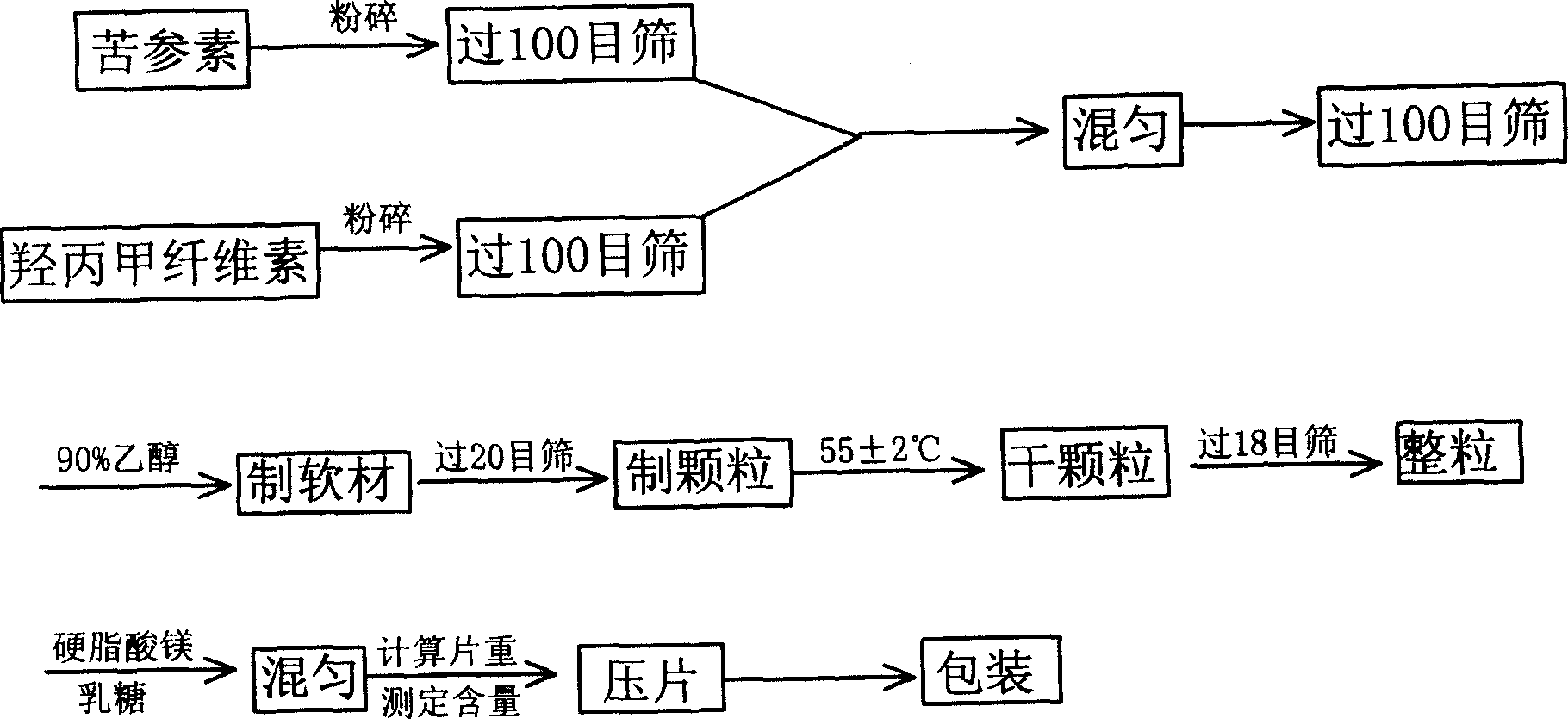
[0056] Such as figure 1 As shown, matrine, hypromellose, lactose, and magnesium stearate were crushed separately, and passed through a 100-mesh sieve for later use; according to the above weight ratio, matrine and hypromellose were fully mixed, passed through 100 Mesh sieve; add 90% ethanol solution of above-mentioned weight ratio to make soft material in matrine and hypromellose after sieving; make wet granule to soft material through 20 mesh sieves; wet granule in 55 Dry at ±2°C; pass the dried granules through a 18-mesh sieve for granulation; add the above-mentioned weight ratio of magnesium stearate and lactose to the sized raw materials for mixing; measure the granules of the mixed raw materi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com