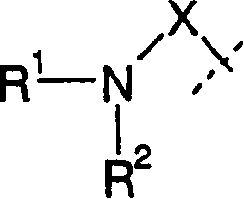
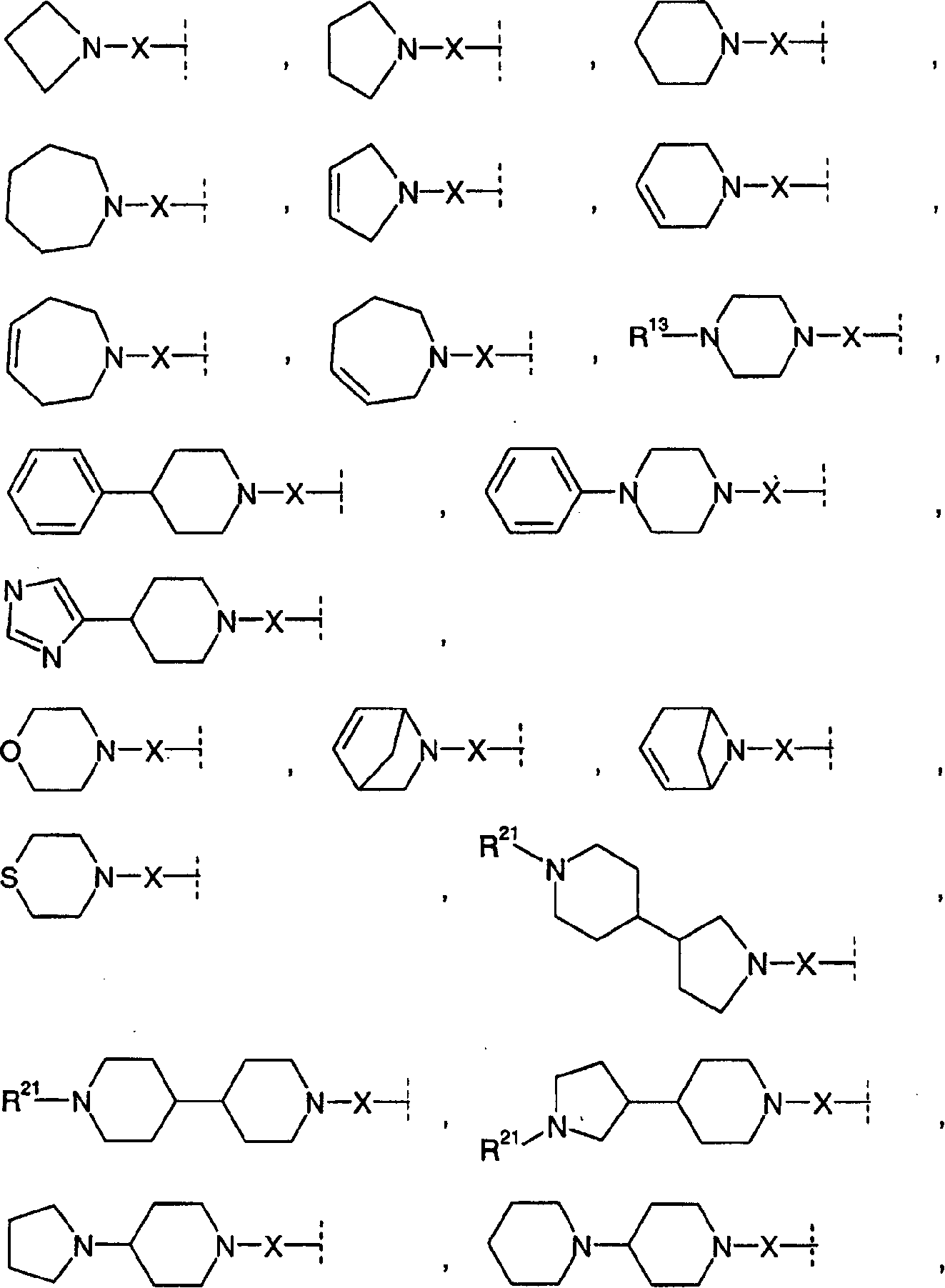
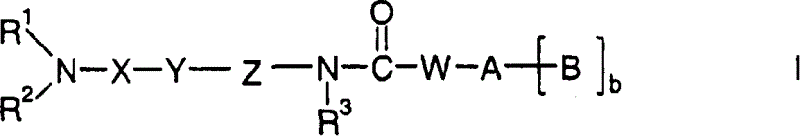Amide compounds having MCH-antagonistic activity and medicaments comprising these compounds
A technology of amide compounds and mixtures, which is applied in the field of novel amide compounds and can solve problems such as the unmentioned compounds having MCH-antagonistic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0541] Synthesis of Intermediates
[0542] Intermediate 1:
[0543]
[0544] Z1a) [2-(2-Chloro-4-nitro-phenoxy)-ethyl]-diethyl-amine-hydrobromide
[0545] Add 40.00 g (1.00 mol) of potassium carbonate to 50.00 g (0.288 mol) of 2-chloro-4-nitro-phenol with 60.23 g (0.350 mol) of (2-chloro-ethyl)-diethyl-amine at 700 mL of DMF, and the mixture was stirred at 80°C for 16 hours. The reaction mixture was evaporated down i. vac, the residue was combined with water, and the aqueous phase was exhaustively extracted with EtOAc. The combined organic extracts were washed with water, dried over magnesium sulfate and evaporated down i. vac. The crude product was recrystallized from EtOAc, and the mother liquor was evaporated down in vacuo. The residue was purified by column chromatography (silica gel, gradient dichloromethane / MeOH 10:0→9:1) to give the desired product.
[0546] Yield: 29.00 g (37% of theory)
[0547] C 12 h 17 ClN 2 o 3 (M=272.734)
[0548] Calculated value...
Embodiment Z23f
[0938] Prepared similarly to Example Z23f from (4-piperidin-1-ylmethyl-phenyl)-acetonitrile. Yield: 1.4 g (85.9% of theory)
[0939] C 14 h 22 N 2 (M=218.34)
[0940] Calculated value: molecular peak (M+H) + :219
[0941] Measured value: Molecular peak (M+H) + :219
[0942] R f Value: 0.2 (silica gel, dichloromethane / ethanol / ammonia 20:1:0.1)
[0943] Intermediate 25:
[0944]
[0945] Z25a) Ethyl 5-hydroxy-2-nitrobenzoate
[0946] A solution of 5.00 g (27.304 mmol) of 5-hydroxy-2-nitrobenzoic acid in 200 mL of ethanol-containing HCl was refluxed for 5 hours and then stirred at room temperature for 48 hours. The reaction mixture was evaporated to dryness in vacuo and diluted with EtOAc. The organic phase is washed with water, dried over magnesium sulfate and evaporated down i. vac. The crude product was used in the next reaction step without any further purification.
[0947] Yield: 5.00 grams (87% of theory)
[0948] C 9 h 9 NO 5 (M=211.176)
[0949] Cal...
Embodiment 19
[1329]
[1330] 19) 2-(2-Chloro-4-trifluoromethyl-phenoxy)-N-[4-(2-diethylamino-ethoxy)-3-methoxy-phenyl]-B Amide
[1331] 171 mg (0.82 mmol) CDI was added to a solution of 185 mg (0.73 mmol) (2-chloro-4-trifluoromethyl-phenoxy)-acetic acid (see intermediate Z2b) in 5 mL THF , and the reaction mixture was stirred at 50 °C for 30 minutes. Then, 0.1 ml (0.73 mmol) of triethylamine and 200 mg (0.73 mmol) of 4-(2-diethylamino-ethoxy)-3-methoxy-aniline (see intermediate Z6b) were added, And the solution was stirred at room temperature for 16 hours. The reaction solution was added to water, and stirred at room temperature for 45 minutes. After filtration, the residue was dried in a circulating air drier.
[1332] Yield: 170 mg (49% of theory)
[1333] C 22 h 26 CIF 3 N 2 o 4 (M=474.912)
[1334] Calculated value: molecular peak (M+H) + : 475 / 477
[1335] Measured value: Molecular peak (M+H) + : 475 / 477(Cl)
[1336] R f Value: 0.30 (silica gel, dichloromethane / MeOH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com