Lipid microspherical asarol prepn and its prepn process
A technology of octylolipid and lipid microspheres, which is applied in the field of asaronelolipid microsphere preparation and its preparation, to achieve the effect of reducing the amount of normal tissue distribution, reducing the dosage, and overcoming the instability of chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
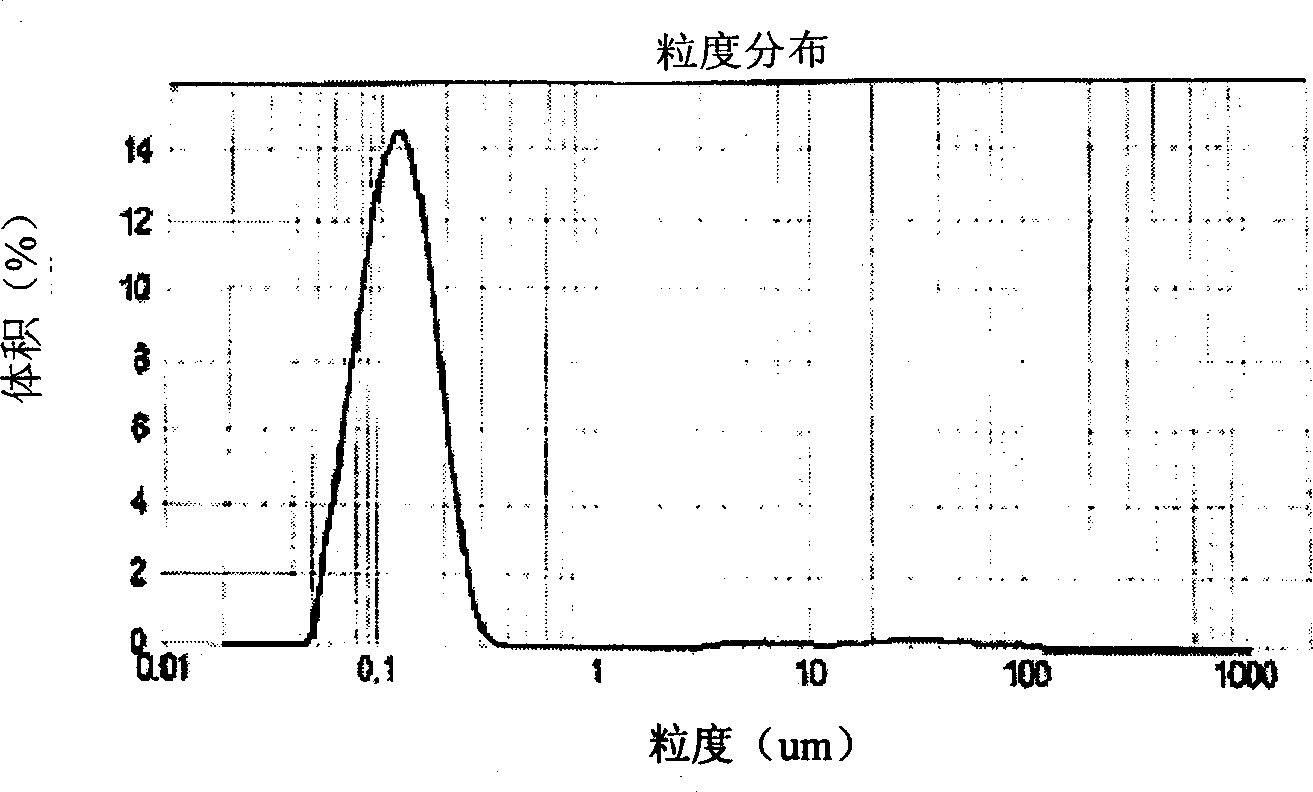
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of lipid microsphere preparation of the present invention
[0034]Put 1.5 g of soybean lecithin for injection and 2.5 g of glycerol for injection into a high-speed tissue masher, add an appropriate amount of water for injection, and prepare a uniform dispersed phase at a speed of 12000 rpm; mix the dispersed phase with Asarone 240 10 milligrams of oil for injection is preheated to 60-80°C, mixed to make colostrum, and the colostrum is added to water for injection to 100 milliliters, placed in a high-pressure homogenizer for repeated emulsification, and filtered through a 3-micron microporous membrane , and then filled with nitrogen and filled in an ampoule or an infusion bottle, and sterilized to obtain the lipid microsphere preparation for intravenous injection, which can also be taken orally.
Embodiment 2
[0035] Example 2 Preparation of lipid microsphere preparation of the present invention
[0036] Soybean lecithin for injection 1.2g and pronic (F-68) 0.3g and water for injection are first prepared into a uniform dispersed phase; add preheated 10 milliliters of oil for injection that is dissolved with 480 mg of asarone to make colostrum, Add water for injection to make up to 100 ml; emulsify under high pressure, filter through a 0.8-micron microporous membrane, fill it in an ampoule or an infusion bottle with nitrogen filling, and sterilize to obtain a lipid microsphere preparation for intravenous injection.
Embodiment 3
[0037] Example 3 Preparation of lipid microsphere preparation of the present invention
[0038] 1.5g of soybean lecithin for injection, 2.5g of glycerol for injection and water for injection are first prepared into a uniform dispersed phase; 20 ml of oil for injection that is dissolved with 240 mg of asarone in preheating is added to make colostrum, and water for injection is mixed to 100 ml, high-pressure emulsification, and filling with nitrogen to prepare intravenous lipid microsphere preparation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com