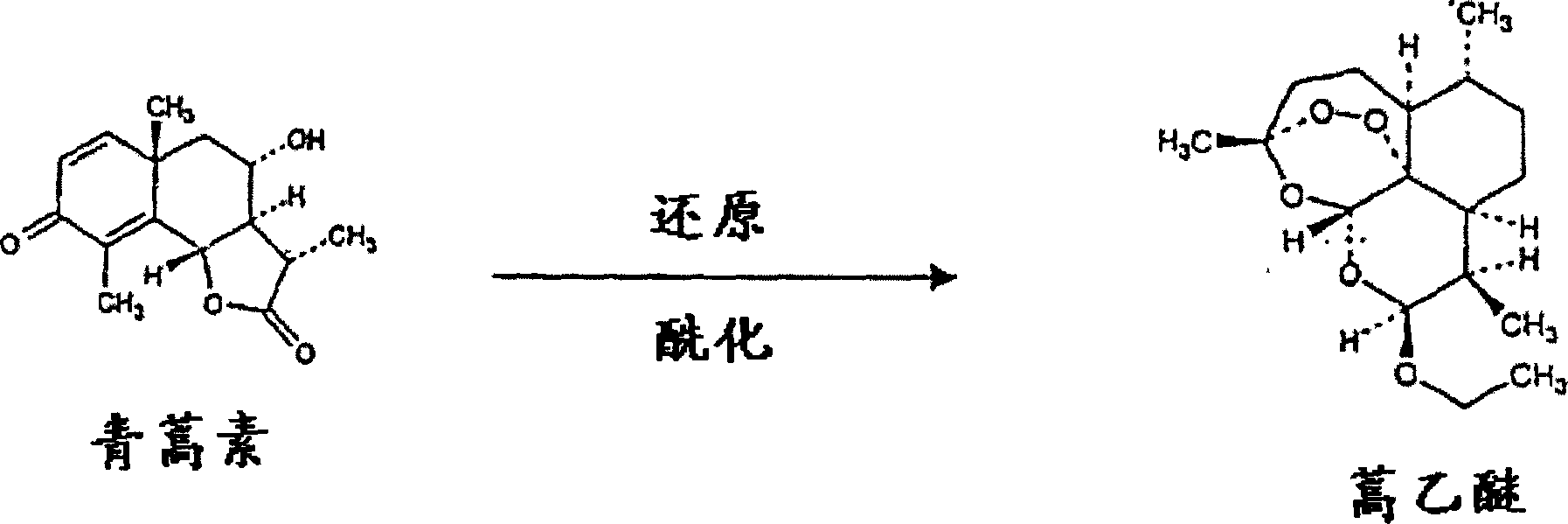
Single pot conversion of artemisinin into arteether
A kind of arteether and artemisinin technology, which is applied in the field of single-pot method of converting artemisinin into artemisinin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Artemisinin (1 g) and polyhydroxy catalyst dextrose (5 g) were stirred in ethanol (20 ml) for 5 minutes at room temperature. At this point sodium borohydride (600 mg) was added slowly over 10 minutes and the reaction mixture was stirred at room temperature (20 to 23°C) for about 1 hour. The reaction was monitored by TLC to check the completion of the reduction step. The acid catalyst trimethylchlorosilane (3.5ml) was slowly added at 10-23°C, and the reaction mixture was stirred at room temperature for about 1 hour. Cold water (about 150ml) was added to the reaction mixture and the aqueous reaction mixture was extracted with 1% ethyl acetate in hexane (3 x 50ml).
[0056] The combined ethyl acetate-hexane extracts were washed with 0.5% sodium bicarbonate solution (100 mL) and then with water (50 mL). The n-hexane extract was dried over anhydrous sodium sulfate and the solvent was evaporated to obtain 1.038 g of crude artether with some impurities. The impure artemethe...
Embodiment 2
[0058] Artemisinin (1 g) and polyhydroxy catalyst dextrose (4 g) were stirred in ethanol (15 ml). Sodium borohydride (500 mg) was added slowly over 10 minutes, and the reaction mixture was stirred at room temperature (20 to 23° C.) for 30 minutes. After the reduction step was complete, trimethylchlorosilane (3.5 ml) was added and the reaction mixture was stirred at room temperature for an additional 1.5 hours. After conventional work-up and purification by column chromatography (1:5 ratio), a mixture of alpha and beta arteether (0.805 g, 80.5% w / w) was obtained.
Embodiment 3
[0060] Artemisinin (1 g) and polyhydroxy catalyst dextrose (2 g) were stirred in ethanol (25 ml). Sodium borohydride (700 mg) was added slowly over 10 minutes, and the reaction mixture was stirred at room temperature (20 to 23° C.) for 1.5 hours. After the reduction step was complete, chlorotrimethylsilane (4 ml) was added and the reaction mixture was stirred at room temperature (20 to 23° C.) for an additional 2 hours to obtain 0.95 g of crude artether. After conventional work-up and purification by column chromatography, 0.95 g of crude artether yielded 0.825 g of a mixture of alpha and beta artether (82.5% w / w).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com