Reduction pressure peptide derived from bee milk
A technology of royal jelly and high blood pressure, which is applied in the field of food and preparations for oral intake, achieving the effect of small side effects and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0365] (1) Preparation of protein breakdown product of royal jelly raw material
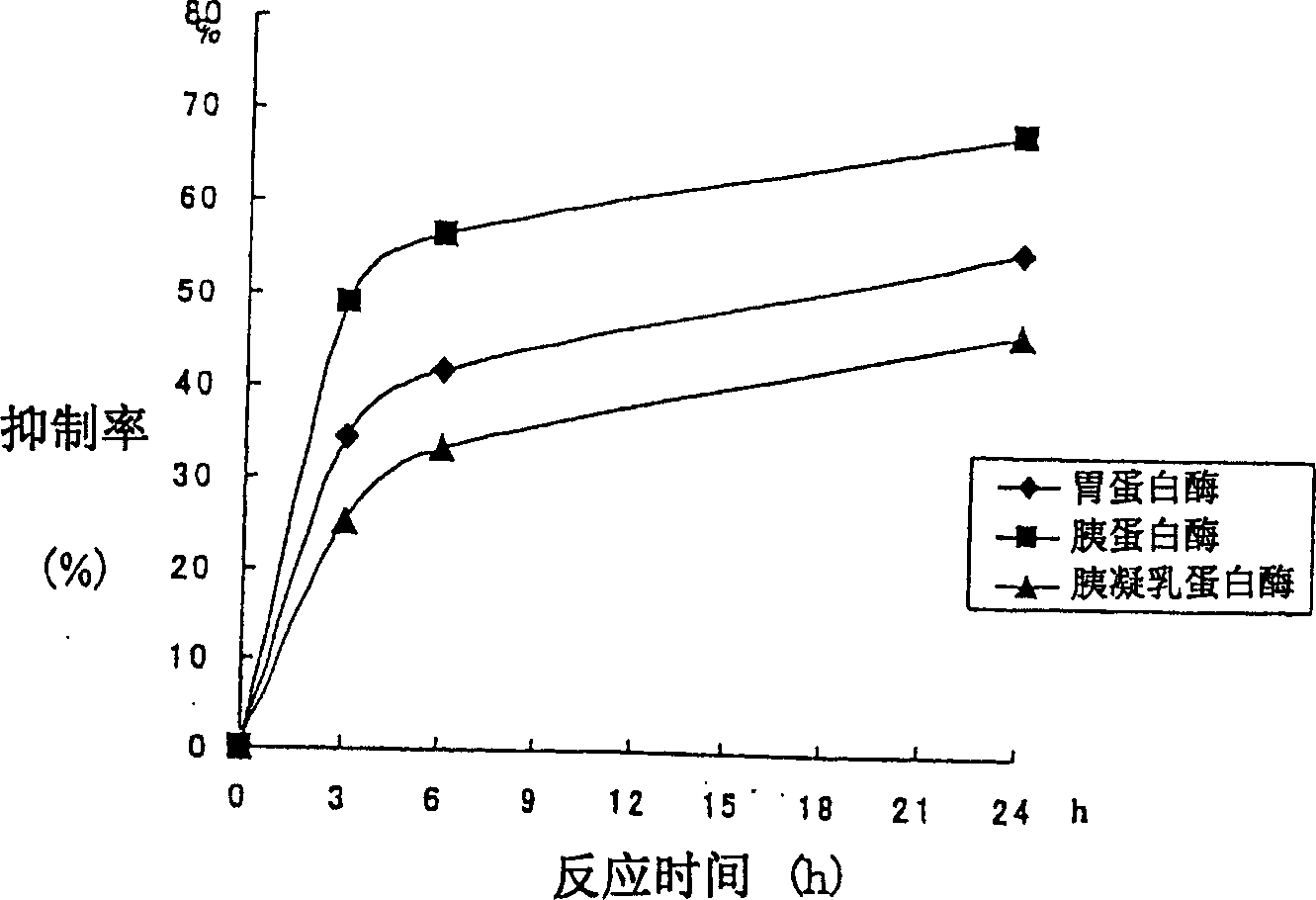
[0366] 1 g of dried royal jelly (protein content 0.4 g) was dissolved in 10 ml of distilled water to adjust the pH to 8, then 0.25% by weight of trypsin (derived from bovine pancreas, manufactured by Roche) was added to the royal jelly raw material, and incubated for 24 hours. The ACE inhibition rate was measured for the samples after 3 hours, 6 hours, and 24 hours from the start of incubation. The pH of the reaction solution was maintained at around pH 8 by adding sodium hydroxide.
[0367]Use chymotrypsin (pH7) or pepsin (pH2) instead of trypsin, and incubate for 24 hours in the same way, and measure ACE inhibition according to the following (2) for the samples after the initial incubation for 3 hours, 6 hours, and 24 hours Rate.
[0368] (2) Determination of ACE inhibitory activity
[0369] 25 μl of the protein breakdown product obtained above at 4 mg / mL (concentration of the reaction solut...
Embodiment 2
[0404] The ethanol-denatured protein of royal jelly precipitated by adding ethanol to raw royal jelly was used instead of 1 g of dry royal jelly (protein content 0.4 g), and the same treatment as in Example 1 was carried out for 24 hours. The ACE inhibition rate of the trypsin-treated product was obtained, and the ACE inhibition rate almost equal to that of the royal jelly of Example 1 was obtained.
[0405] The reaction solution treated with trypsin for 24 hours was adjusted to pH 4.5, loaded onto a synthetic adsorbent (Sepabizu SP-70 (manufactured by Mitsubishi Chemical)), washed with water, and sodium chloride and acidic or basic free amino acids were removed. and sugars, etc., were eluted with ethanol to obtain the desalted protein hydrolyzate of the present invention.
[0406] For the dried royal jelly (dry RJ) used in Example 1, the trypsin hydrolyzate obtained in Example 1 before desalination (before desalination; dry RJ-enzyme), and the ethanol-denatured protein of roy...
Embodiment 3
[0421] Embodiment 3: Structural analysis of the relevant components of royal jelly (RJ) protein hydrolyzate
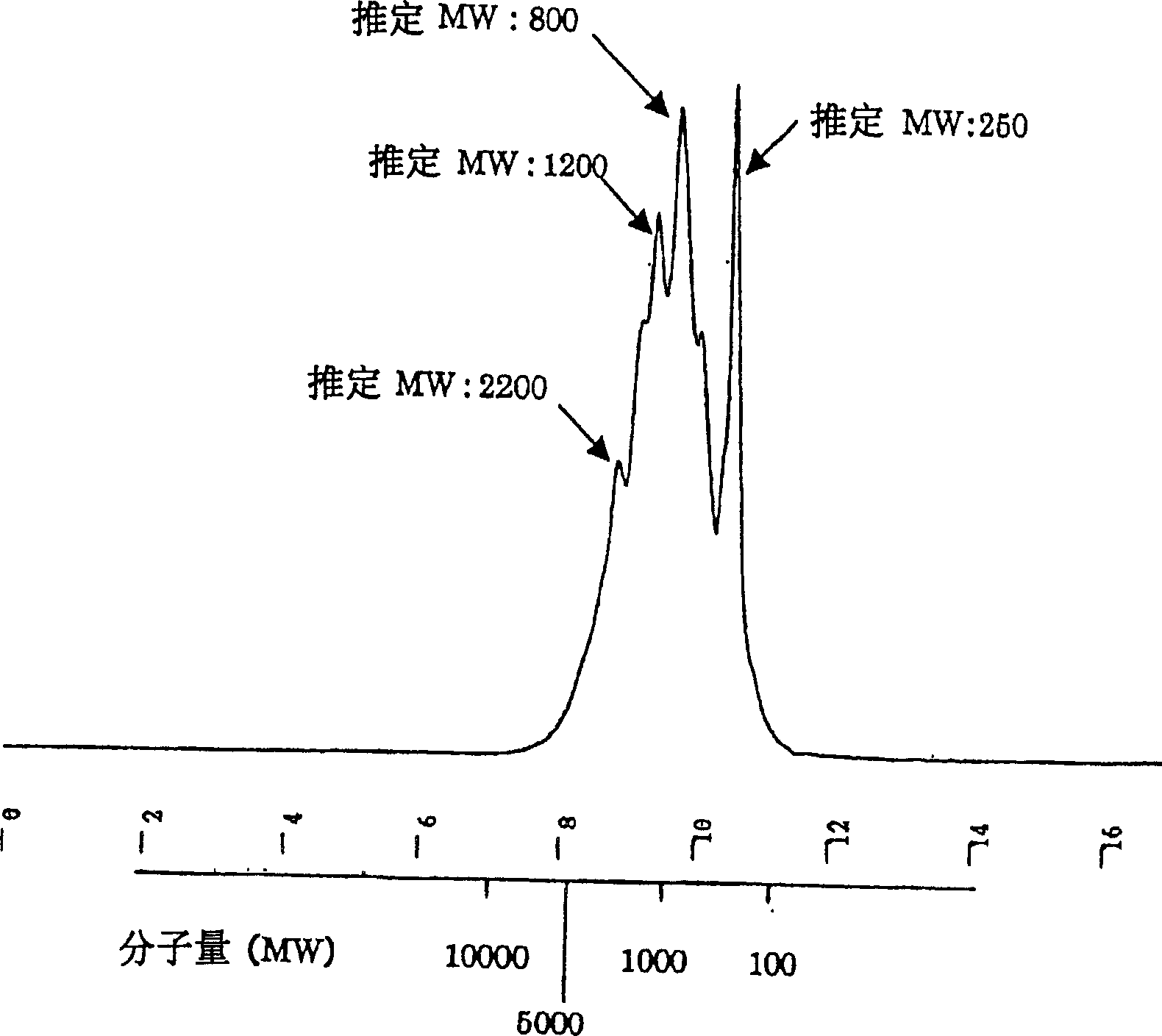
[0422] Take 100 g of ethanol-denatured protein of royal jelly, according to the method described in Example 1, under the condition of pH 8.0, use trypsin to carry out enzymatic decomposition at 37° C. for 24 hours. Heat the reaction solution in a boiling water bath for 30 minutes to inactivate the enzyme, then adjust the pH to 4.5 with hydrochloric acid, centrifuge (10000rpm, 20 minutes), and filter the obtained supernatant with a membrane filter to obtain RJ proteolysis Material 1160mL.
[0423] The obtained RJ protein hydrolyzate was eluted with Diayion HP20 according to the above-mentioned route, with water, 20% methanol, 40% methanol, 60% methanol, 80% methanol and methanol (100%) to obtain fraction 1 (Fr1) to Point 7 (Fr7).
[0424] Then, the details of the isolation and identification of the 9 related components (peptides) identified from fractions 3-6 are listed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com