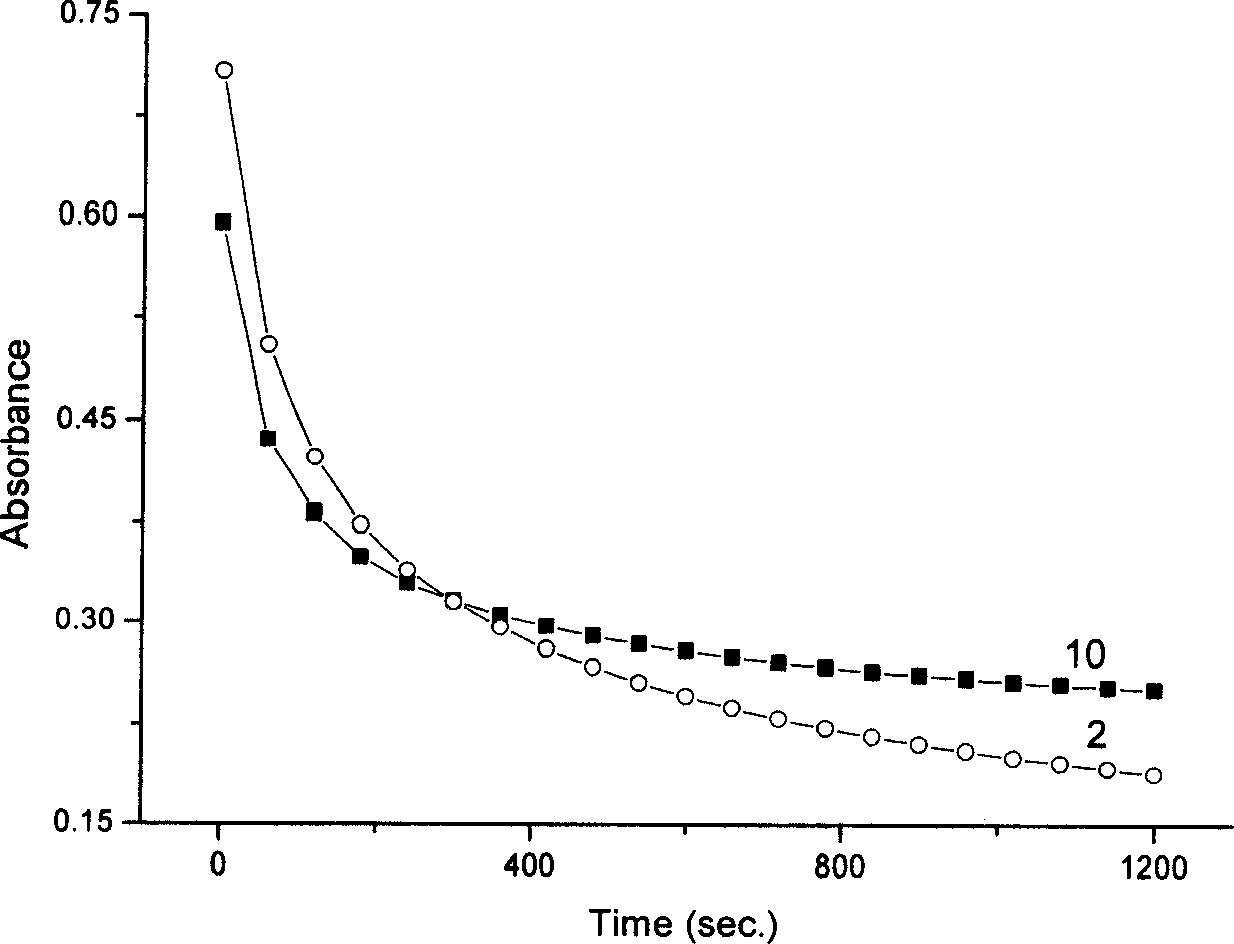
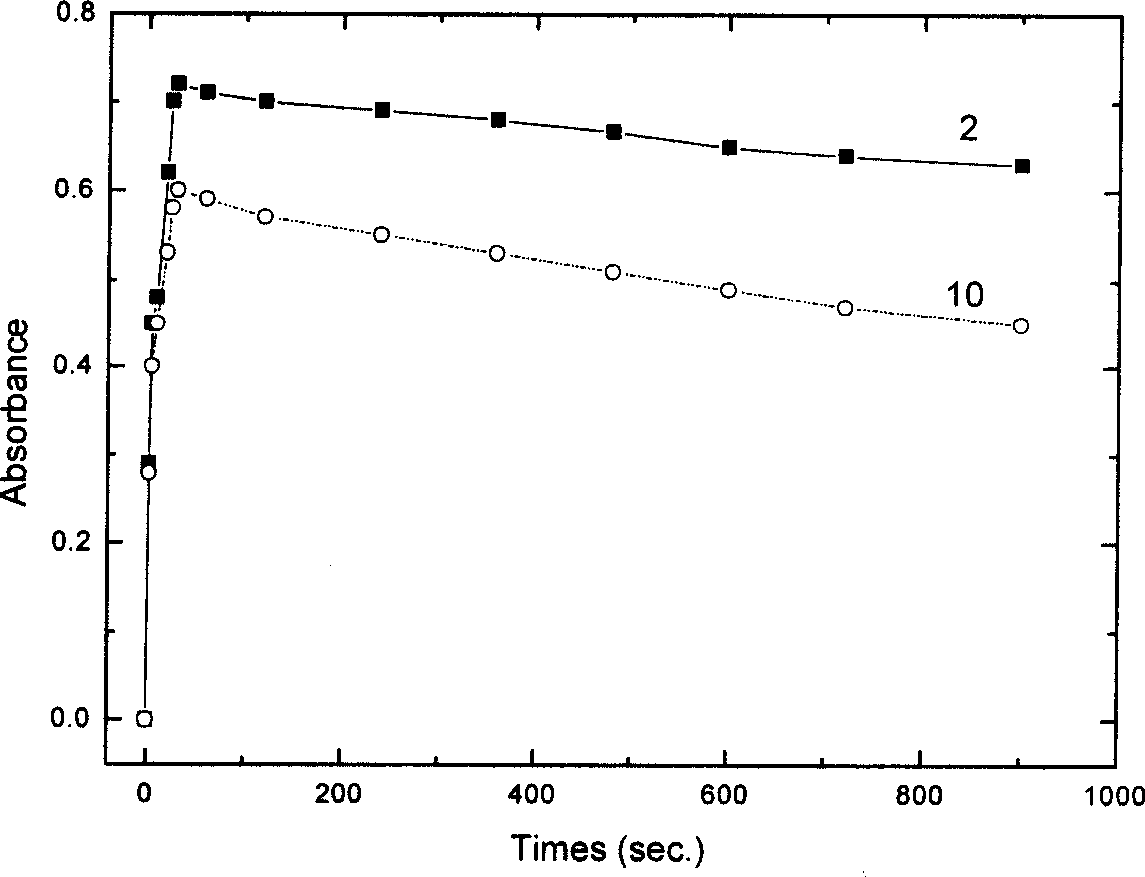
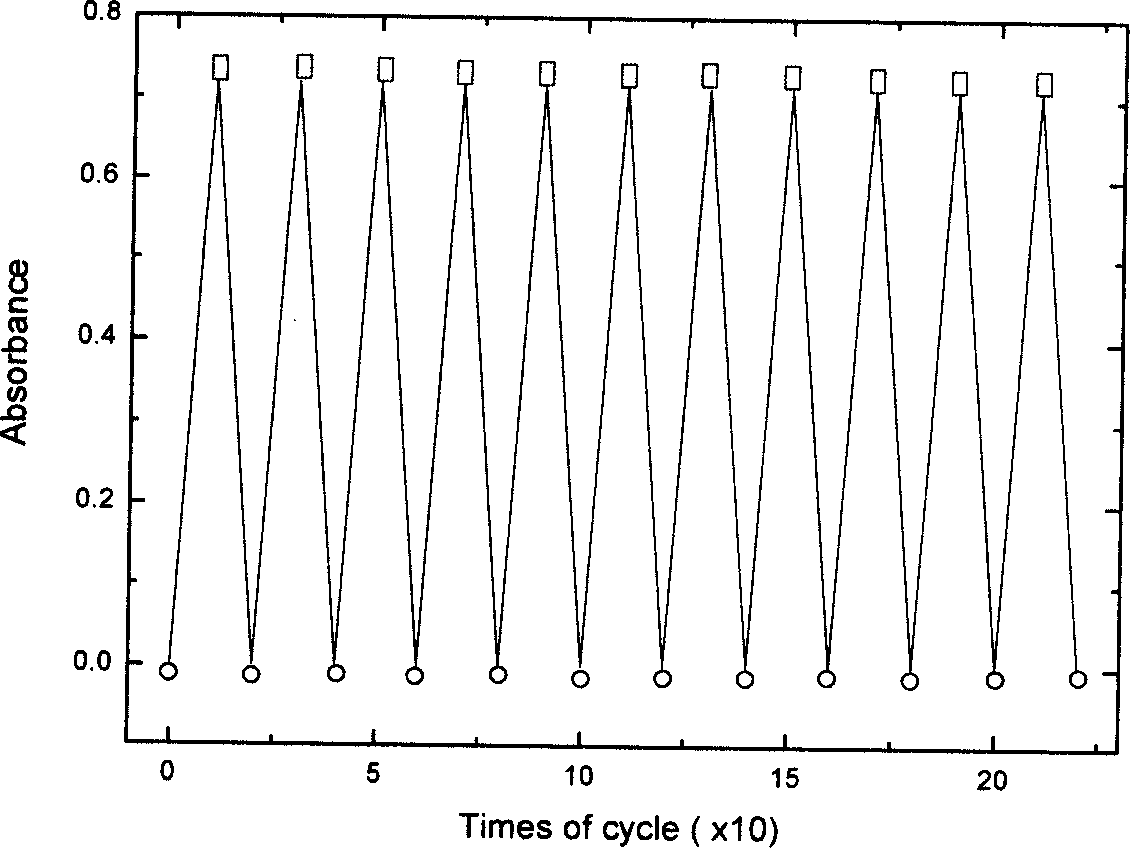
Nano composite materials and products of photochromism of heterocyclic ring substituted spirooxazine compounds
A nano-composite material, photochromic technology, applied in the direction of color-changing fluorescent materials, optics, optical components, etc., can solve the problems of failure to dissolve the photochromic material, affecting the defoaming, bonding strength, and increasing the processing cost, etc. Excellent photochromic performance, adjustable fading rate, fast fading effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] For the preparation of photochromic compounds 1-6, take the preparation of 5-chloro-1,3,3-trimethyl-6'-morpholine-spirooxazine (compound 1) as an example. The preparation methods of compounds 2-6 are similar. Compounds 1-6 are listed in Table 1.
[0046] Preparation of 5-chloro-1,3,3-trimethyl-6'-morpholine-spirooxazine (compound 1)
[0047] The first step, the preparation of 5-chloro-1,3,3-trimethyl-2-methylene indoline
[0048] In a 1000mL three-neck flask, add 26g of p-chloroaniline and 150mL of 6M HCl, heat to dissolve, then cool it to about 0°C with an ice bath, control the temperature and add dropwise 15g of NaNO 2 60mL of aqueous solution, continue to stir for 30 minutes, filter, add Na to the filtrate 2 SO 3 solution (70g Na 2 SO 3+400mL water), after the addition, stir again until the temperature rises to room temperature, add concentrated HCl to acidify. The solid was obtained by suction filtration, and after drying thoroughly, weighed 35 g of the solid...
Embodiment 2
[0059] Blue (1,3,3-trimethyl-6'-indoline-spiroindoline-2,3'-[3H]naphtho[2,1-b][1,4]oxazine) Preparation of Photochromic PMMA Sunglasses for Radiation Protection:
[0060] Weigh 930g of PMMA resin powder and add it into 5580mL of anhydrous toluene, heat and stir, and dissolve to obtain a colorless transparent solution. To this solution was added 1,3,3-trimethyl-6'-indoline-spiroindoline-2,3'-[3H]naphtho[2,1-b][1,4]oxa 50g of oxazine photochromic nanocomposite material, 5g of light stabilizer, 5g of oxygen quencher and 10g of antioxidant, after fully stirring and dissolving, pour evenly into a Petri dish and store in a dark room. After the solvent was completely evaporated, it was dried in an oven at 60 °C for 20 min. Then, the membrane is peeled off from the dish. The prepared membranes were kept in a dark room.
Embodiment 3
[0062] Violet (1,3,3-trimethyl-6'-piperidine-spiroindoline-2,3'-[3H]naphtho[2,1-b][1,4]oxazine) radiation protection Preparation of Sunglasses with Photochromic PMMA:
[0063] Weigh 930g of PMMA resin powder and add it into 5580mL of anhydrous toluene, heat and stir, and dissolve to obtain a colorless transparent solution. To this solution was added 1,3,3-trimethyl-6'-piperidine-spiroindoline-2,3'-[3H]naphtho[2,1-b][1,4]oxazine 50g of photochromic nano-composite material, 5g of light stabilizer, 5g of oxygen quencher and 10g of antioxidant, after fully stirring and dissolving, pour evenly into a Petri dish and store in a dark room. After the solvent was completely evaporated, it was dried in an oven at 60 °C for 20 min. Then, the membrane is peeled off from the dish. The prepared membranes were kept in a dark room.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com