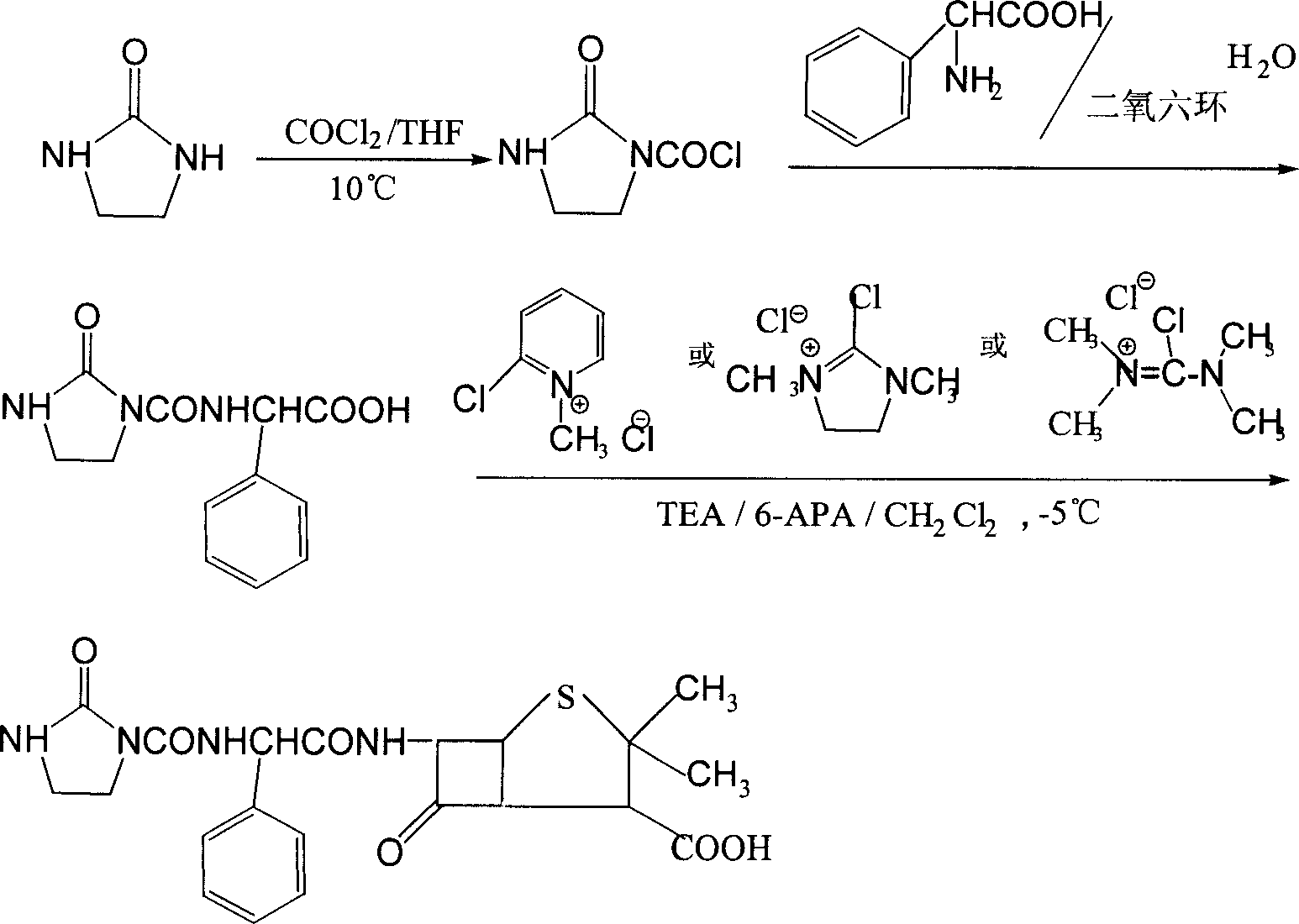
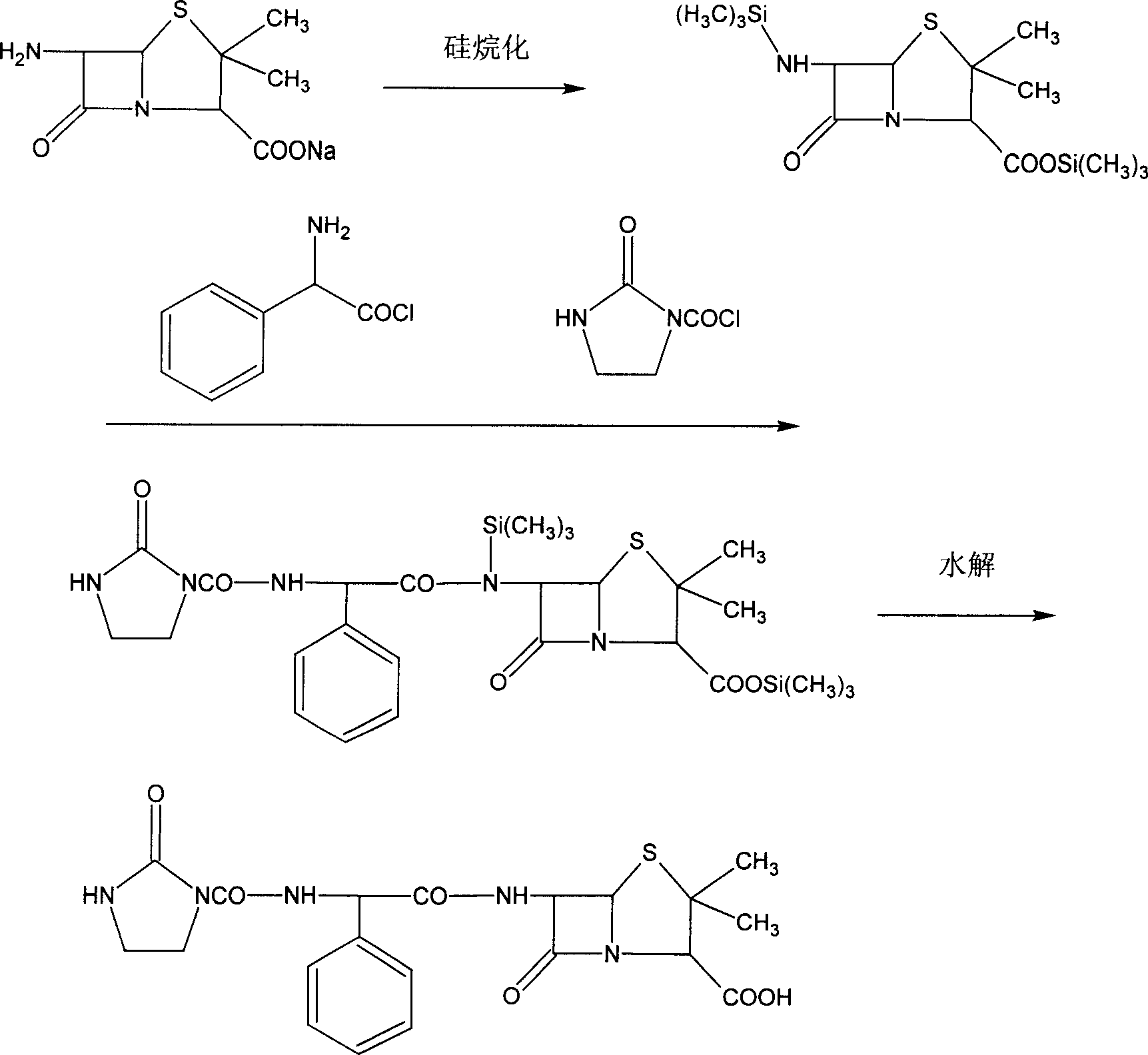
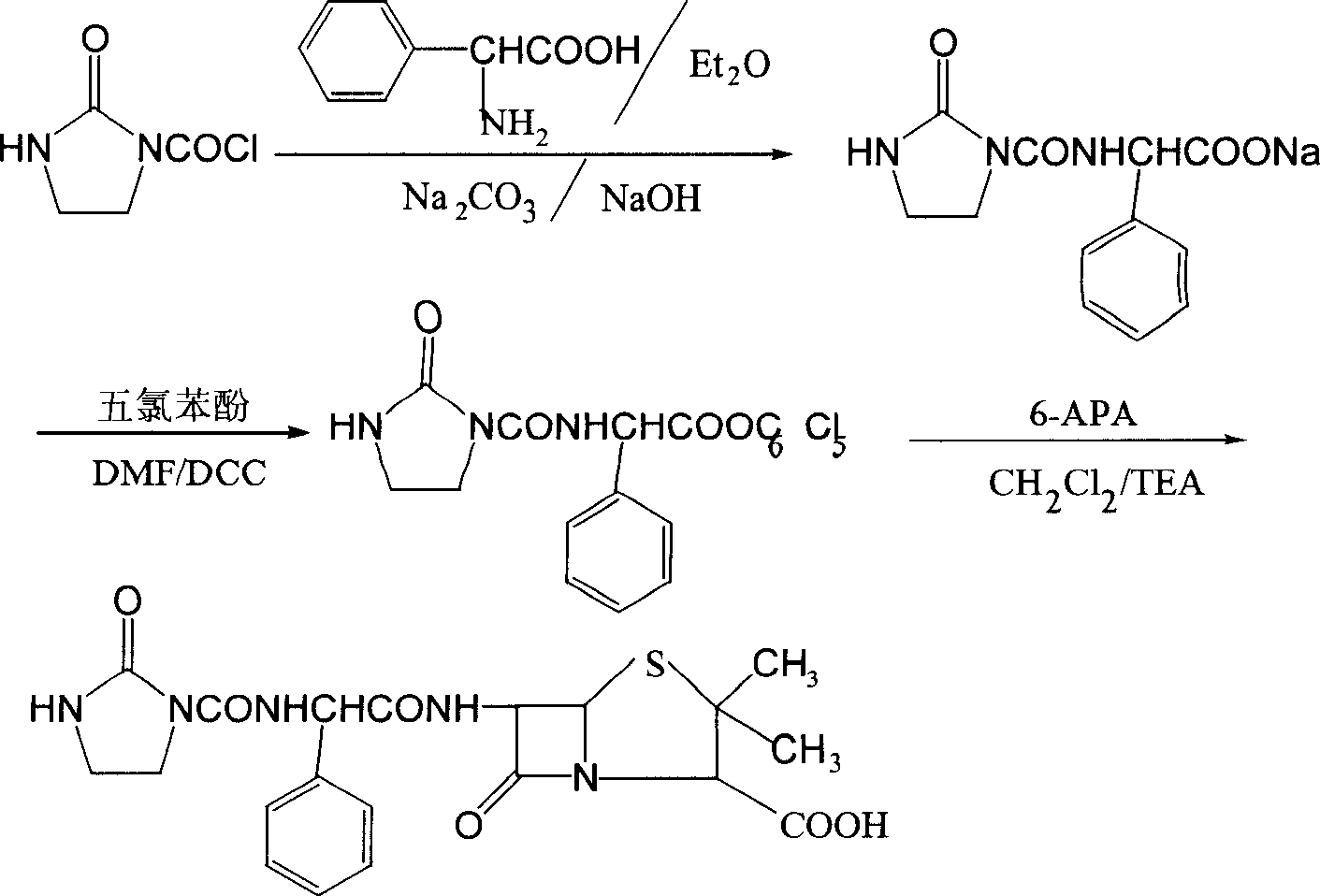
Process for preparing sodium azlocillin
A technology of azlocillin sodium and ampicillin, which is applied in the field of drug preparation, can solve the problems of long method steps, harsh reaction conditions, and high operating conditions, and achieve the effect of solving the problem of "three wastes" treatment, mild reaction conditions, and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Raw material
[0043] raw material name
Feeding amount
molar weight
The molar ratio of
Remark
1400g
3.47
1.05
Deionized water
14L
490g
3.3
1
506mL
3.47
1.05
17L
368g
2.3L
1.47L
ethyl acetate
6L
Sodium isooctanoate
608.5g
3.64
1.1
ethyl acetate
5L
Washed products
[0044] (2) Process steps
[0045] Add 14 liters of water into a 50-liter reaction tank, cool to 4°C, add 1400g of ampicillin while stirring, slowly add 506ml of triethylamine dropwise at 4°C, and finish the dropwise addition in about half an hour, continue to stir for half an ho...
Embodiment 2
[0049] (1) Raw material
[0050] raw material name
Feeding amount
molar weight
The molar ratio of
Remark
1467g
3.64
1.10
Deionized water
14L
490g
3.3
1
530mL
3.64
1.10
ethyl acetate
17L
368g
2.3L
1.47L
ethyl acetate
6L
Sodium isooctanoate
636.2g
3.83
1.15
ethyl acetate
5L
Washed products
[0051] (2) Process steps
[0052] Add 14 liters of water into a 50-liter reaction tank, cool to 0°C, add 1467g of ampicillin while stirring, slowly add 530ml of triethylamine dropwise at 0°C, continue stirring for half an hour, and then the reaction solution becomes cle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com