Recombinant human granulocyte colony stimulating factor and one-step purifying process for chemical modifications thereof
A technology of colony-stimulating factor and granulocytes, applied in the direction of cytokines/lymphokines/interferons, chemical instruments and methods, animal/human proteins, etc. Activity effects and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0015] Example 1 rhG-CSF construction, expression and renaturation
[0016] The amino acid sequence of human G-CSF is as follows:
[0017] NH 2 -Met Thr Pro Leu Gly Pro Ala Ser Ser Leu Pro Gln Ser Phe Leu Leu LysCys Leu Glu Gln Val Arg Lys Ile Gln Gly Asp Gly Ala Ala Leu Gln Glu Lys Leu CysAla Thr Tyr Lys Leu Cys His Pro Glu Glu Leu Val Leu Leu Gly His Ser Leu Gly IlePro Trp Ala Pro Leu Ser Ser Cys Pro Ser Gln Ala Leu Gln Leu Ala Gly Cys Leu SerGln Leu His Ser Gly Leu Phe Leu Tyr Gln Gly leu Leu Gln Ala Leu Glu Gly Ile SerPro Glu Leu Gly Pro Thr Leu Asp Thr Leu Gln Leu Asp Vla Ala Asp Phe Ala Thr ThrIle Trp Gln Gln Met Glu Glu Leu Gly Met Ala Pro Ala Leu Gln Pro Thr Gln Gly AlaMet Pro Ala Phe Ala Ser Ala Phe Gln Arg Arg Ala Gly Gly Val Leu Val Ala Ser HisLeu Gln Ser Phe Leu Glu Val Ser Tyr Arg Val Leu Arg His Leu Ala Gln Pro-COOH
[0018] Whole-gene synthesis of human G-CSF cDNA gene fragments, the use of recursive PCR method to obtain the 3' end containing NdeI and 5' end ...
example 4
[0043] The one-step purification of example four alkylation method PEG-G-CSF
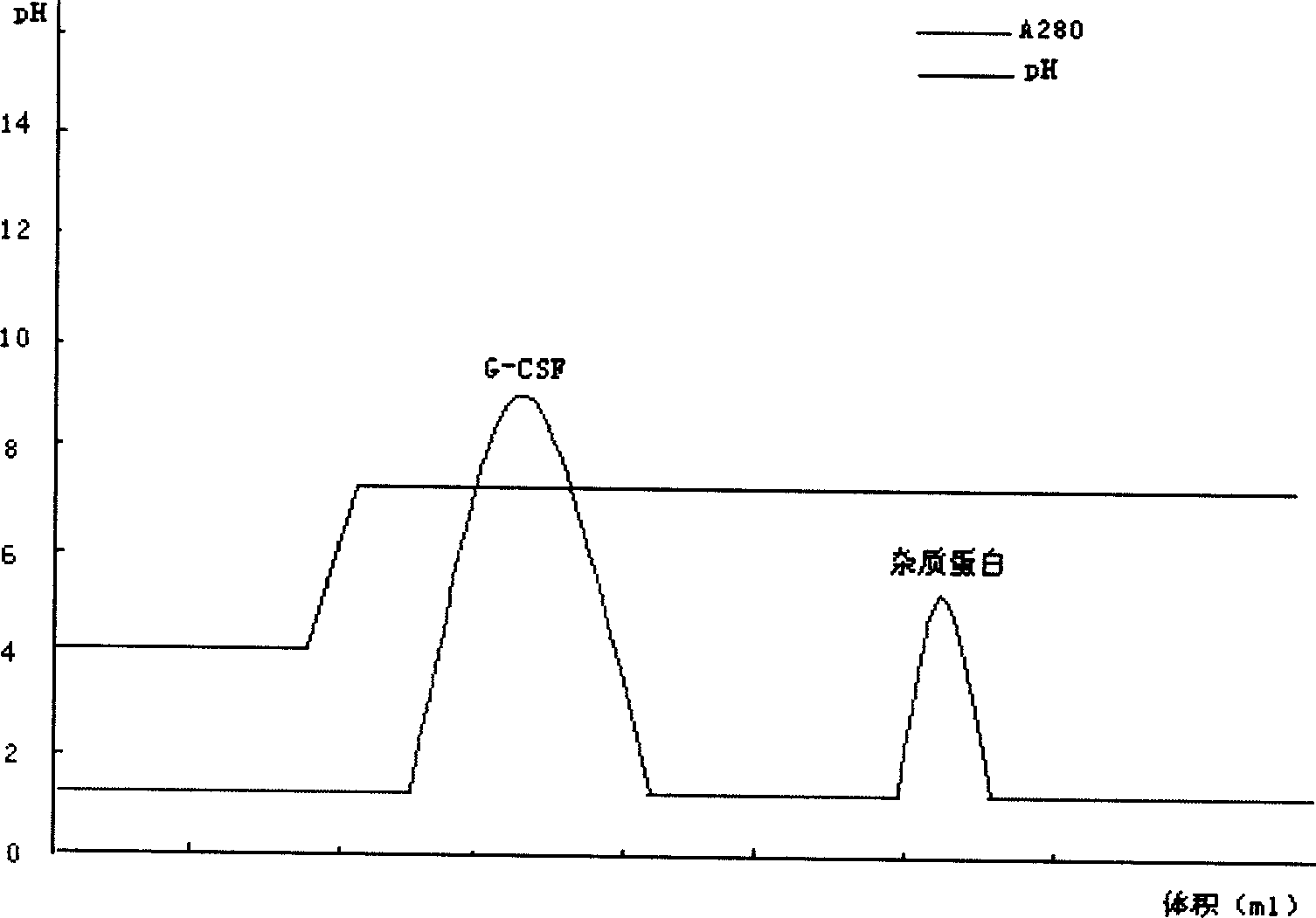
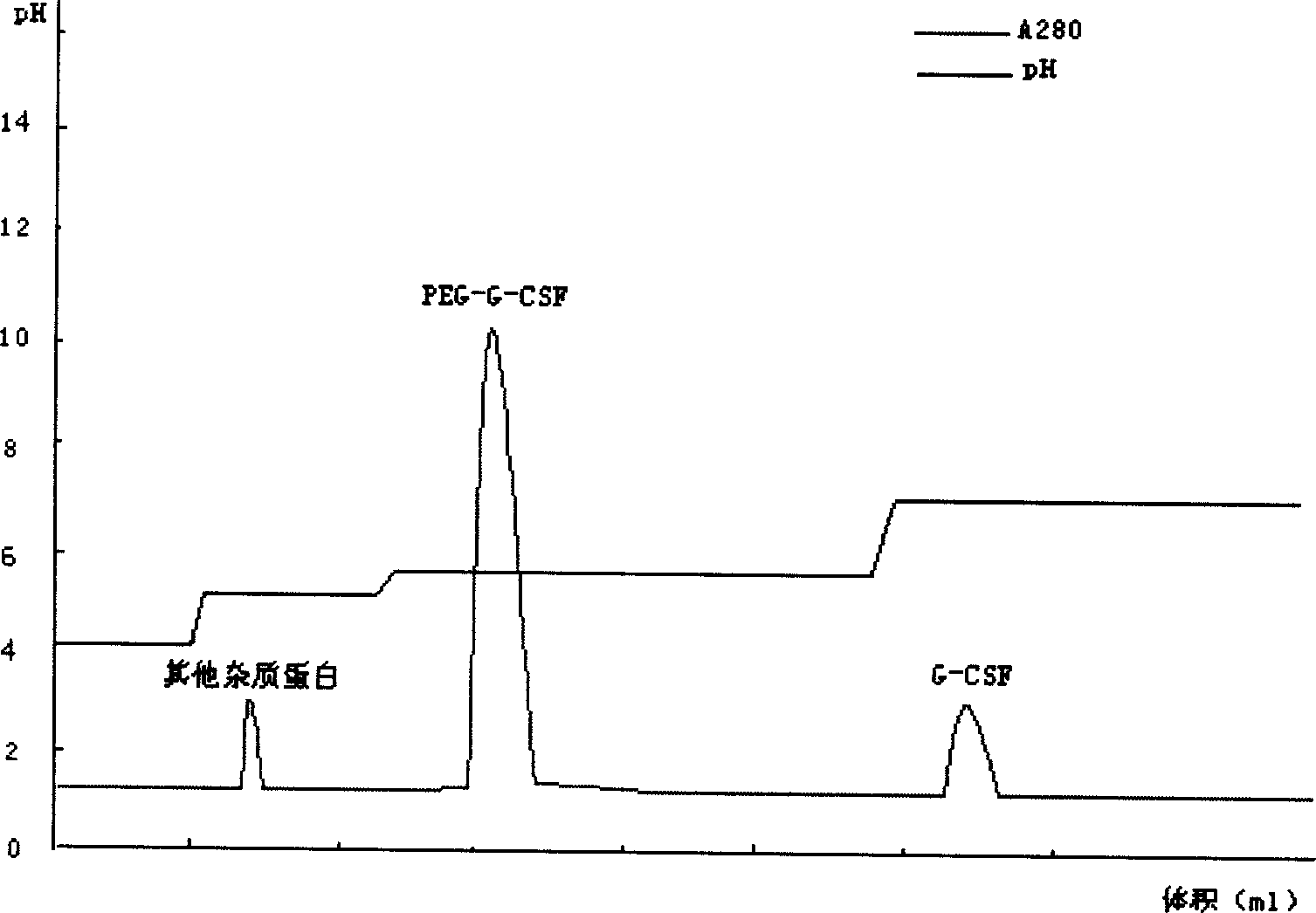
[0044] For sample treatment, take the modified sample, dilute it to twice the volume with distilled water, adjust the pH to 4.0 with acetic acid, and centrifuge to remove the precipitate. Take the cation chromatography medium SP Sepharose F.F., take the equilibration buffer I (10mmol / L acetic acid-sodium acetate, pH4.0) after loading the column to equilibrate to the baseline, then load the sample, and then use the equilibration buffer II (10mmol / L acetic acid- sodium acetate, pH 5.0) equilibrated to baseline. Take elution buffer I (10mmol / L acetic acid-sodium acetate, pH5.6) to elute the PEG-G-CSF target protein peak. Take elution buffer II (10mmol / L acetic acid-sodium acetate, pH7.0) to elute the unmodified rhG-CSF protein peak.
example 5
[0045] One-step purification of example five acylation method PEG-G-CSF
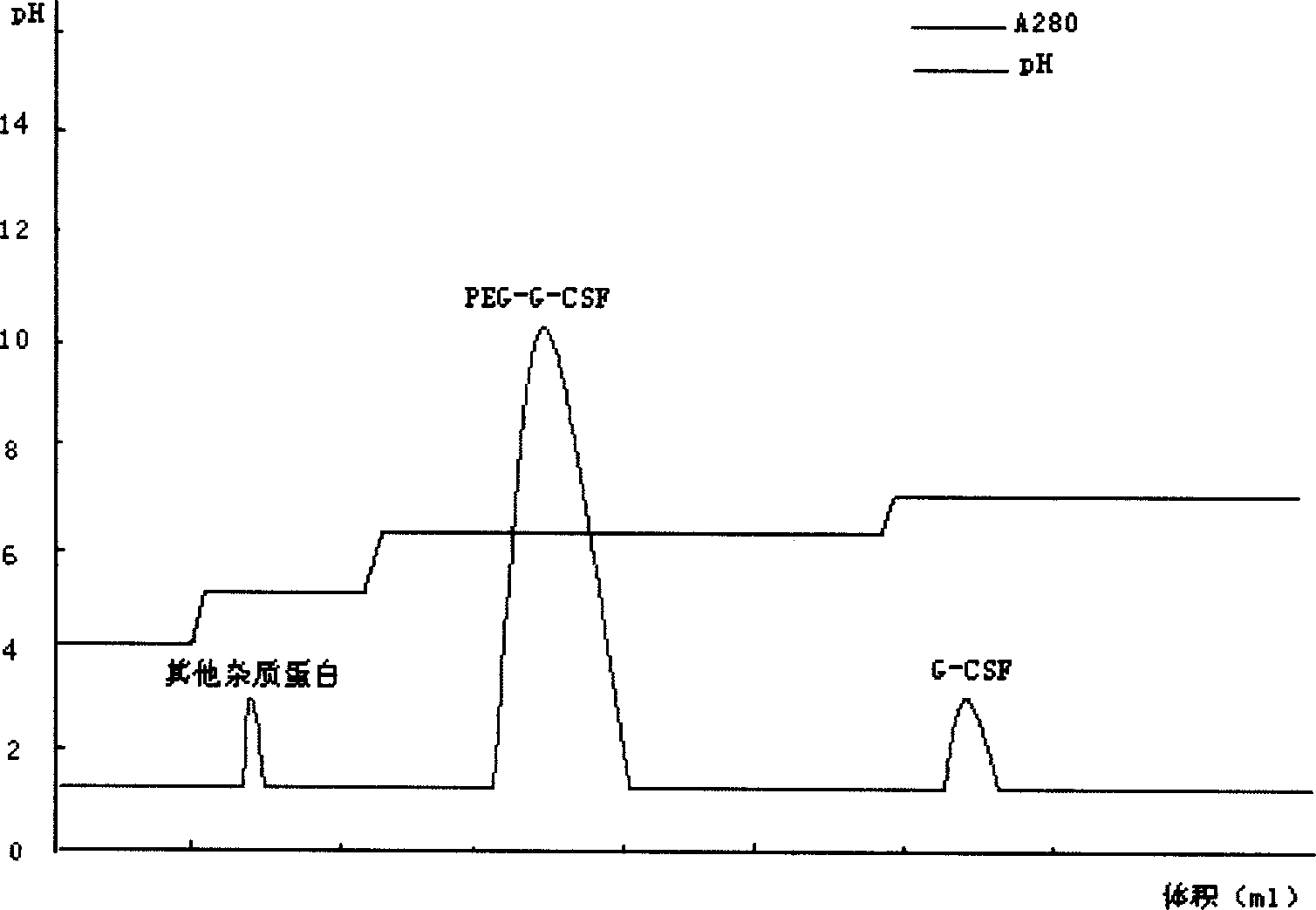
[0046] For sample treatment, take the modified sample, dilute it to twice the volume with distilled water, adjust the pH to 4.0 with acetic acid, and centrifuge to remove the precipitate. Take the cation chromatography medium SP Sepharose F.F., take the equilibration buffer I (10mmol / L acetic acid-sodium acetate, pH4.0) after loading the column to equilibrate to the baseline, then load the sample, and then use the equilibration buffer II (10mmol / L acetic acid- sodium acetate, pH 5.0) equilibrated to baseline. Take elution buffer I (10mmol / L acetic acid-sodium acetate, pH6.2) to elute the PEG-G-CSF target protein peak. Take elution buffer II (10mmol / L acetic acid-sodium acetate, pH7.0) to elute the unmodified rhG-CSF protein peak.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com