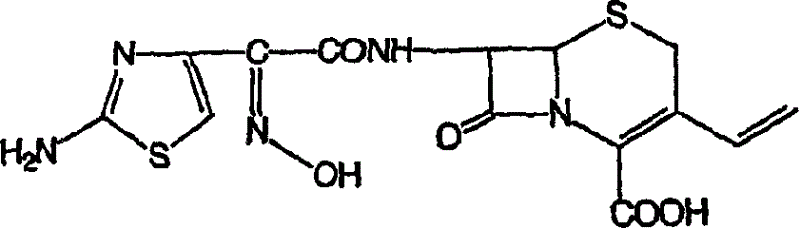
Process for prepn. of cefdinir
A technology for cefdinir and a compound is applied in the field of industrial-scale preparation of cefdinir, and can solve problems such as difficulty in large-scale production, cumbersome post-processing operation steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
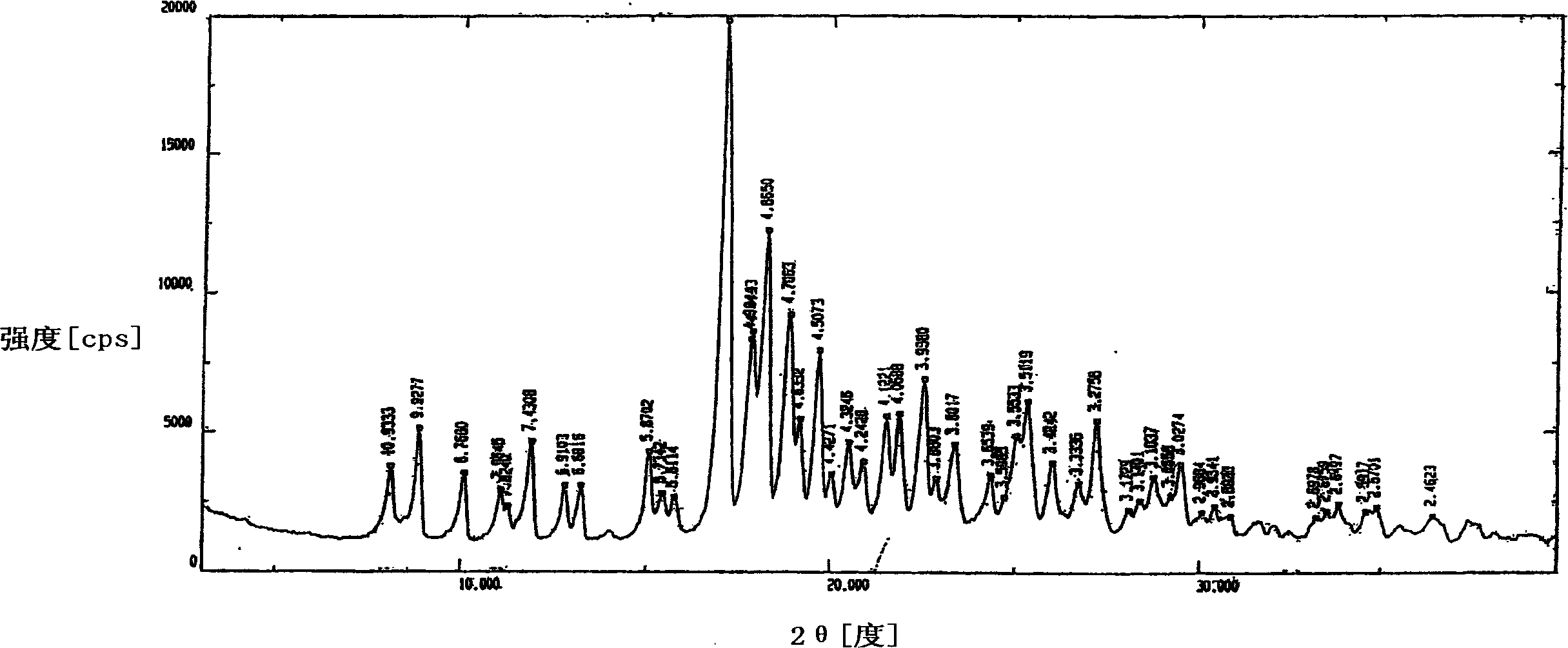
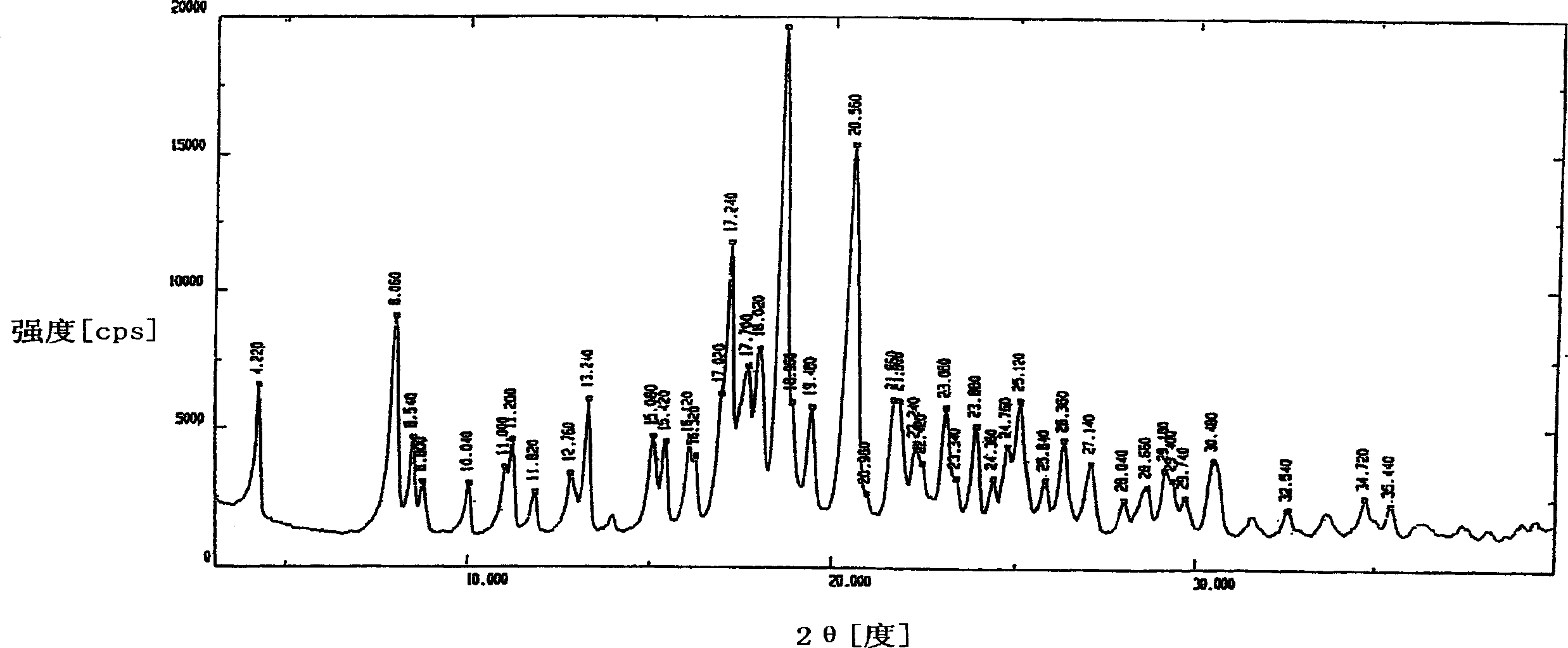
Image
Examples
Embodiment 1
[0056] 7β-[2-(2-Aminothiazol-4-yl)-2(Z)-trityloximino-3-vinyl-3-cephem-4-carboxylic acid, sulfate, 3N,N- Dimethylacetamide solvate
[0057]7-amino-3-vinyl-3-cephem-4-carboxylic acid (10g) was added to N,N-dimethylacetamide (100ml), and then 2-benzothiazolyl (Z)- 2-(2-Aminothiazol-4-yl)-2-trityloxyiminothioacetate (28.2 g). The reaction mixture was cooled to 10-15°C and tri-n-butylamine (17.2 g) was added at 10-15°C over 20-30 minutes. The reaction mixture was stirred at room temperature for 6-7 hours to complete the reaction. Thereafter, it was cooled to -10°C, and sulfuric acid (13.4 g) was added dropwise within 30 minutes below 0°C. Under cooling, toluene (100 ml) was added to the reaction mixture, followed by hexane (100 ml). The temperature of the reaction mixture was raised to 35-40°C and crystallization proceeded. The temperature was maintained at 35-40°C for 30 minutes. The precipitate thus obtained was filtered and washed with toluene, and then dried to obtain 41...
Embodiment 2
[0065] 7β-[2-(2-aminothiazol-4-yl)-2(Z)-(trityloxyimino)acetamido]-3-vinyl-3-cephem-4-carboxylic acid, methyl Sulfonate, 3N,N-Dimethylacetamide solvate
[0066] 7-amino-3-vinyl-3-cephem-4-carboxylic acid (10g) was added to N,N-dimethylacetamide (150ml), and then 2-benzothiazolyl (Z)- 2-(2-Aminothiazol-4-yl)-2-trityloxyiminothioacetate (26.8 g). At 10-15°C, tri-n-butylamine (16.78 g) was added to the reaction mixture. The reaction mixture was stirred at room temperature for 7-8 hours to complete the reaction. Anhydrous methanesulfonic acid (13 g) was added to the reaction mixture over 15-20 minutes at below 10°C, followed by diisopropyl ether (150 ml). The temperature of the reaction mixture was raised back to 30-35°C, and crystallization proceeded. The precipitate thus obtained was filtered and washed with diisopropyl ether, and then dried to obtain 38.5 g (yield: 96%) of the title compound as off-white crystals.
[0067] HPLC purity: 99.3%, m.p.=125-127°C, N,N-dimethylac...
Embodiment 3
[0072] 7β-[2-(2-aminothiazol-4-yl)-2(Z)-(trityloxyimino)acetamido]-3-vinyl-3-cephem-4-carboxylic acid, methyl Sulfonate, 2N,N-Dimethylacetamide solvate
[0073] 7-amino-3-vinyl-3-cephem-4-carboxylic acid (15g) was added to N,N-dimethylacetamide (225ml), and then 2-benzothiazolyl (Z)- 2-(2-Aminothiazol-4-yl)-2-trityloxyiminothioacetate (45 g). At 10-15°C, tri-n-butylamine (27 g) was added to the reaction mixture. The reaction mixture was stirred at 25-30°C for 7-8 hours to complete the reaction. Anhydrous methanesulfonic acid (210 g) was added to the reaction mixture over 15-20 minutes at below 10°C, followed by diisopropyl ether (450 ml). The temperature of the reaction mixture was raised back to 38-40°C and stirred for 45 minutes for crystallization. The suspension was cooled to 25-30°C and stirred for a further 1 hour. The precipitate thus obtained was filtered and washed with diisopropyl ether, and then dried to obtain 56.7 g (yield: 94.2%) of the title compound as off...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com