Synthesis of water soluble oxamonoamide
A technology for the synthesis of oxamonoamides, which is applied to the preparation of carboxylic acid amides, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as low yield, limited industrial application, and difficult separation, and achieve high yield , The effect of easy product and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
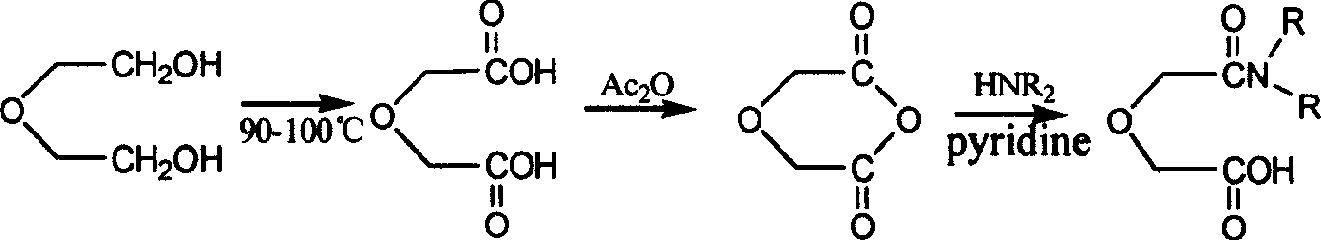
[0008] The invention proposes a synthesis method of water-soluble oxamonoamide. The method uses diethylene glycol as a raw material, undergoes oxidation and condensation to obtain diglycolic anhydride, and then reacts with dialkylamine to obtain amide. Its reaction formula is as follows:
[0009]
[0010] The synthesis method is divided into three steps:
[0011] (1) diethylene glycol prepares diglycolic acid: diethylene glycol is oxidized to diglycolic acid through concentrated nitric acid (concentration is 10mol / L), (the volume ratio of diethylene glycol and concentrated nitric acid is 1: 7~10);
[0012] (2) Diglycolic acid generates diglycolic anhydride: Diglycolic acid and acetic anhydride (molar ratio is 1:5~7) are reacted and crystallized with toluene to obtain white diglycolic anhydride crystals; Dosage plus toluene;
[0013] (3) Synthesis of oxamonoamide: Diglycolic anhydride is reacted with dimethylamine, diethylamine or dipropylamine (molar ratio is 1:1) respect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com