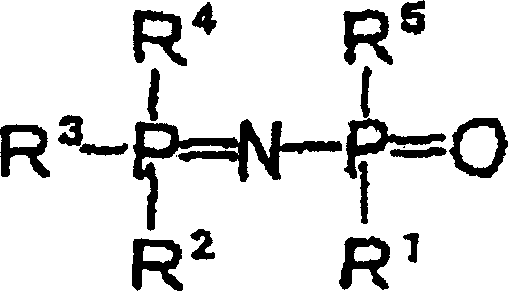
Additive for non-aqueous liquid electrolyte, non-aqueous liquid electrolyte secondary cell and non-aqueous liquid electrolyte electric double layer capacitor
A technology for electrolyte additives and electric double-layer capacitors, which is applied in the direction of non-aqueous electrolyte batteries, non-aqueous electrolyte batteries, electrolytic capacitors, etc., and can solve safety problems, non-aqueous electrolyte fire, and the danger of electrolyte flame burning expansion sexuality issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] [Preparation of non-aqueous electrolyte solution]
[0192] Add (20 % by weight) phosphazene derivative 20g (in the above general formula (1), R is a methoxyl group, n is a cyclic phosphazene derivative of 3) (non-aqueous electrolyte additive), and then LiBF 4 (Supporting salt) was dissolved at a concentration of 0.75 mol / kg to prepare a non-aqueous electrolyte (25°C viscosity: 8.2mPa·s (8.2cP), conductivity of 0.75mol / l lithium salt solution: 6.5mS / cm) .
[0193] In addition, the above-mentioned viscosity and electrical conductivity were measured by the above-mentioned measuring methods, respectively.
[0194]
[0195] The obtained non-aqueous electrolytic solution was evaluated as follows in the same manner as the above-mentioned evaluation method of self-extinguishing property and flame retardancy, and the results are shown in Table 1.
[0196] "Evaluation of incombustibility"
[0197] The ignited flame does not reach 25mm of the device, and it is not considered...
Embodiment 2
[0217] In the "preparation of non-aqueous electrolytic solution" of Example 1, except for 70 g of a mixed solvent of diethyl carbonate and ethane carbonate, and 30 g (30% by weight) of a phosphazene derivative, it is the same as in Example 1 Non-aqueous electrolyte solution (viscosity at 25°C: 9.7mPa·s (9.7cP), conductivity of 0.75mol / l lithium salt solution: 5.8mS / cm), self-extinguishing property, non-combustibility, and degradation resistance sexual evaluation. Furthermore, non-aqueous electrolyte storage batteries were prepared in the same manner as in Example 1, and the initial battery characteristics (voltage, internal resistance), charge-discharge cycle performance, and low-temperature characteristics were measured and evaluated, respectively. The results are shown in Table 1.
Embodiment 3
[0219]In the "preparation of non-aqueous electrolytic solution" of Example 1, in addition to 94.5 g of mixed solvents of diethyl carbonate and ethane carbonate, 5.5 g (5.5% by weight) of phosphazene derivatives, and LiPF 5 In place of the supporting salt, prepare the same non-aqueous electrolyte solution as in Example 1 (the viscosity at 25°C is 3.7mPa·s (3.7cP), the conductivity of the lithium salt solution of 0.75mol / l: 7.4mS / cm), Evaluations were performed from self-extinguishing properties to incombustibility and deterioration resistance. Furthermore, non-aqueous electrolyte storage batteries were prepared in the same manner as in Example 1, and the initial battery characteristics (voltage, internal resistance), charge-discharge cycle performance, and low-temperature characteristics were measured and evaluated, respectively. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com